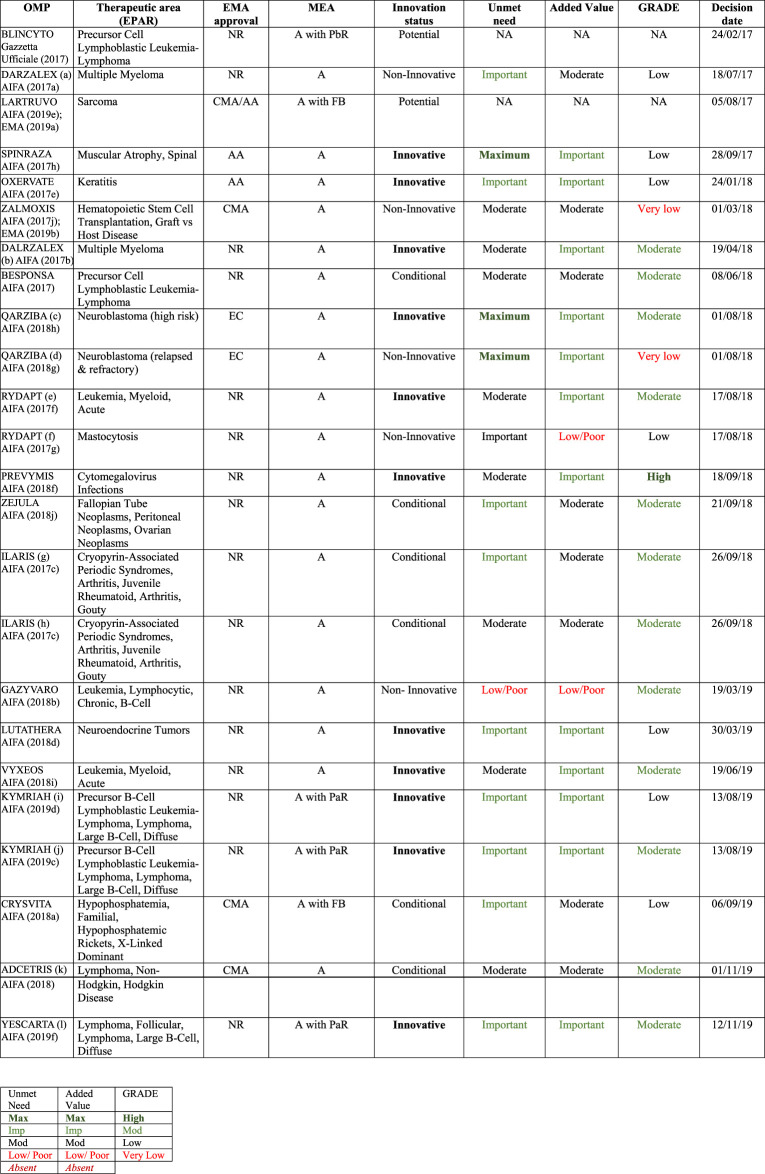
TABLE 2.
OMPs registries and innovation status (2017–2019 analysis).
|
AA, accelerate assessment; CMA, conditional marketing authorisation; CTS, Commissione Tecnico-Scientifica; EC, exceptional circumstances; NR, normal route.
Registry closed.
For the treatment of relapsed and refractory multiple myeloma, whose previous therapies have included a proteasome inhibitor and an immunomodulator, and which have shown disease progression during the last therapy.
For the indication in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of adult patients with multiple myeloma who have received at least previous therapy.
High-risk neuroblastoma in patients from 12 months of age who have previously undergone induction chemotherapy achieving at least a partial response, followed by myeloablative therapy and stem cell transplantation.
Patients with history of relapsed or refractory neuroblastoma, with or without residual disease.
Only in combination with standard induction chemotherapy with daunorubicin and cytarabine and consolidation with high-dose cytarabine for adult patients with newly diagnosed acute myeloid leukemia (AML) with positive FLT3 mutation.
Monotherapy for the treatment of adult patients with aggressive systemic mastocytosis (ASM), systemic mastocytosis with associated haematological neoplasm (SM AHN), or mast cell leukaemia (MCL).
Periodic autoinflammatory fever syndromes in adults, adolescents and children from 2 years of age: hyperimmunoglobulin D syndrome (HIDS)/mevalonate kinase deficiency (MKD) and Familial Mediterranean Fever (FMF).
Periodic autoinflammatory fever syndromes in adults, adolescents and children from 2 years of age: Tumor necrosis factor receptor-associated periodic syndrome (TRAPS).
Diffuse large B-cell lymphoma (DLBCL) in adults whose cancer has come back or did not respond after two or more previous treatments.
B-cell acute lymphoblastic leukaemia (ALL), in children and young adults up to 25 years of age whose cancer did not respond to previous treatment, has come back two or more times, or has come back after a transplant of stem cells.
CD30 positive cutaneous T-cell lymphoma (CTCL).
Relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and Primary mediastinal large B-cell lymphoma (PMBCL), after two or more lines of systemic therapy.
