Abstract
Background
It is a global challenge to enrol and retain paediatric patients in HIV/AIDS care. Attrition causes preventable transmission, stoppable morbidity and death, undesirable treatment outcomes, increased cost of care and drug resistance. Thus, this study intended to investigate the incidence and predictors of attrition among children receiving antiretroviral treatment (ART).
Method
A retrospective follow-up study was conducted among children <15 years who had ART follow-up in Gedeo public hospitals. After collection, data were entered into Epi-data V.4.6, then exported to and analysed using STATA V.14. Data were described using the Kaplan-Meier statistics, life table and general descriptive statistics. The analysis was computed using the Cox proportional hazard regression model. Covariates having <0.25 p values in the univariate analysis (such as developmental stage, nutritional status, haemoglobin level, adherence, etc) were fitted to multivariable analysis. Finally, statistical significance was declared at a p value of <0.05.
Results
An overall 254 child charts were analysed. At the end of follow-up, attrition from ART care was 36.2% (92 of 254), of which 70 (76.1%) were lost to follow-up, and 22 (23.9%) children died. About 8145.33 child-months of observations were recorded with an incidence attrition rate of 11.3 per 1000 child-months (95% CI: 9.2 to 13.9), whereas the median survival time was 68.73 months. Decreased haemoglobin level (<10 g/dl) (adjusted HR (AHR)=3.1; 95% CI: 1.4 to 6.9), delayed developmental milestones (AHR=3.6; 95% CI: 1.2 to 10.7), underweight at baseline (AHR=5.9; 95% CI: 1.6 to 21.7), baseline CD4 count ≤200 (AHR=4.4; 95% CI: 1.6 to 12.2), and poor or fair ART adherence (AHR=3.5; 95% CI: 1.5 to 7.9) were significantly associated with attrition.
Conclusion and recommendation
Retention to ART care is challenging in the paediatrics population, with such a high attrition rate. Immune suppression, anaemia, underweight, delayed developmental milestones and ART non-adherence were independent predictors of attrition to ART care. Hence, it is crucial to detect and control the identified predictors promptly. Serious adherence support and strengthened nutritional provision with monitoring strategies are also essential.
Keywords: HIV, syndrome, therapeutics
What is known about the subject?
Retaining patients receiving antiretroviral treatment (ART) in care is critical for successful ART outcomes. However, it is challenging to enrol and retain paediatric patients on ART care where about 20%–36% of children attrite from ART care. Studies also revealed that attrition from ART service is higher among children than in adults and is higher in resource-constraint settings. Different studies and organisations suggested some strategies and interventions intended to prevent attrition from HIV care programmes.
What this study adds?
More than a quarter (27%) of children were lost to follow-up.
Immunosuppression, anaemia, underweight, delayed developmental milestones and ART non-adherence were independent predictors of attrition to ART care.
Introduction
Despite the improvement in the survival of children infected with the virus following the introduction of highly active antiretroviral treatment (ART), AIDS is one of the world’s most serious public health challenges.1 According to the Joint United Nations Programme on HIV/AIDS(UNAIDS) report, in Ethiopia, by 2018, 690 000 people were living with HIV, 23 000 people newly infected with HIV and 11 000 people died from AIDS-related illnesses.2
Attrition refers to the interruption of ART care and is a composite variable which includes patients lost to follow-up, died and those who stopped ART.3 Retention in ART care is essential to prevent HIV-related morbidity and deaths through early detection and treatment of opportunistic infections. It also helps for early ART initiation, prevention and early detection of treatment failure. Moreover, retaining patients receiving ART in care is critical for successful ART outcomes.4–7 Different studies and organisations suggested some strategies and interventions intended to prevent attrition from HIV care programmes such as nutrition support, ensuring uninterrupted drug supplies, consideration of simple, non-toxic ART regimens, decentralisation of ART care, engaging family role in ART care, strengthening the tracing mechanism and a reduction of indirect costs.8 9
However, it is challenging to enrol and retain paediatric patients in ART care.7 10 ART follow-up interruption following an HIV diagnosis is associated with avoidable morbidity and death, increased cost of care, and preventable HIV transmission and drug resistance.6 Attrition occurred in a speckled incidence ranging from 20% to 36%.11–15 Studies also revealed that attrition from ART service is higher among children than in adults6 16 and is higher in resource-constraint settings.11 17–19
Children may attrite from ART care for several reasons, including limited socioeconomic status, inadequate educational levels (lack of understanding of the need for lifelong care), living away from the ART facility and lack of transport. It may also be associated with providers’ negative attitudes, long waiting times, drug side effects and delayed defaulter-tracing systems, suboptimal adherence and social support (stigma and disclosure-related issues).3 6 11 20 21
While several studies had been conducted on adults taking ART,22–25 we have a paucity of evidence about attrition in children receiving ART, especially in resource-limited settings like Ethiopia. Besides, the definitions used by available studies to declare attrition were inconsistent. For instance, some studies considered only lost to follow-up cases and excluded deaths and defaulters. Some other studies included subjects with no follow-up after the diagnosis of HIV/AIDS, which could be challenging to declare attrition. Hence, it is difficult to draw a generalised country figure though having this problem at the back of paediatric ART. Identifying predictors of attrition could aid for focused planning and implementation of suggested strategies to improve retention. Therefore, this study intended to investigate the incidence and predictors of attrition among children receiving ART.
Methods
Study setting and period
The study was conducted among children aged under 15 years who were attending ART follow-up at ART centres in public hospitals of Gedeo, Ethiopia, by reviewing 6 years (January 2013–December 2019) of ART follow-up data. Gedeo zone is located 1765 m above sea level and 394 km away from Addis Ababa. Gedeo has four hospitals, 36 health centres and 146 health posts. All four hospitals had access to paediatric ART services. The study was conducted from November 2019 to June 2020.
Study design
A hospital-based retrospective follow-up study was employed.
Study population
The study population comprised all HIV/AIDS-infected children less than 15 years of age who were attending ART follow-up at selected hospitals of Gedeo zone, Southern Ethiopia. Those children who had no at least one follow-up visit after confirmation of HIV/AIDS infection and charts with incomplete records were excluded.
Sample size determination and sampling procedure
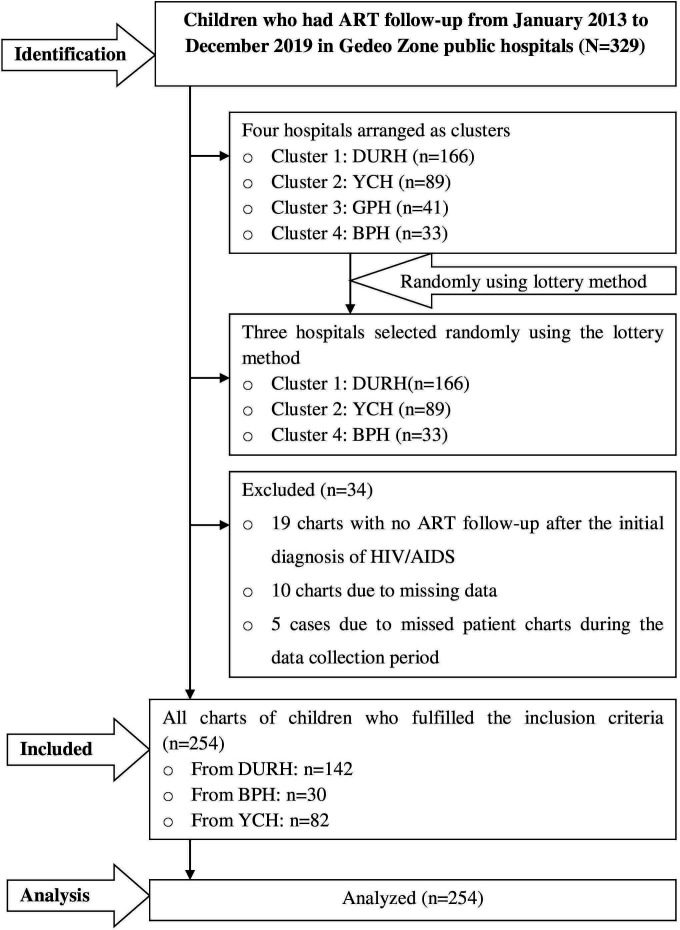
The required sample size was estimated using the log-rank test according to the assumptions of the Cox proportional hazard model by using STATA V.13. Child aged <3 years at the initiation of ART follow-up was identified as a predictor variable from a previous study,21 which yields the maximum sample size. After considering a 95% CI, 80% power, HR=5.3 and probability of failure among the control group (aged 3–8 years)=0.0423, the required sample size was 262. Thus, 262 charts were needed to be incorporated for review to conduct this study. A cluster sampling technique was applied after adjusting the four public hospitals providing ART services as clusters. Then, the three hospitals (Dilla University Referral Hospital, Bule Primary Hospital and Yirga Chefe Primary Hospital) were selected randomly using the lottery method. Afterwards, all charts that had been recorded from January 2013 to December 2019 were included for review. The details of steps and procedure of study participants’ selection were described in the flow chart (figure 1).
Figure 1.
A flow chart of recruitment methods among HIV/AIDS-infected children who had ART follow-up at ART clinics of Gedeo public hospitals, Southern Ethiopia, 2020 (N=254). ART, antiretroviral treatment; BPH, Bule Primary Hospital; DURH, Dilla University Referral Hospital; GPH, Gedeb Primary Hospital; YCH, Yirga Chefe Hospital.
Operational definitions and measurements
Survival time was measured from the beginning of ART follow-up until the time of the event or censoring. Children who had follow-up interruption either due to loss from ART follow-up, death or defaulters were considered as events (attrition),3 6 whereas children who were active on ART follow-up, transferred out to other health institutions and those who exceed 15 years during the follow-up period were recorded as censored. Children were recorded lost if they did not return to the ART visit within 90 days or more after their last scheduled appointment.6 26 Adherence was assessed based on the 2017 national ART score system.6 27 The time scale in this study was in months. Nutritional status was assessed by weight-for-age (WFA) and height-for-age (HFA) using z-score and then classified as underweight (WFA ≤−2 z-score), stunting (HFA ≤−2 z-score) and normal (z-score >2). Severe immunodeficiency was defined as CD4 <200 for children under 5 years of age and CD4 <100 for those aged above 5 years.26 27 The developmental milestone (<5 years) was determined as delayed where the child fails to achieve the expected milestone for age; regression in which the child loses what has been attained for age; and otherwise normal.6 26
Data collection tools and procedures
The authors initially observed patient records and designed an appropriate data extraction checklist in English after reviewing different literature, ART-monitoring chart and the national comprehensive HIV treatment guideline.6 The tool comprised of sociodemographic, clinical, and disease-related and treatment-related variables. The lists of study participants were obtained from the ART data clerk, and charts were selected from the hospital card room using a medical record number. Three data collectors (ART-trained BSc) and one supervisor (MSc fellow) completed the data collection within 4 weeks, from 15 April to 15 May 2020.
Data quality assurance
A week before starting the actual data collection, the principal investigator conducted a pretest on 14 (5%) randomly selected charts at Yirgalem Primary Hospital to assure the consistency and clarity of the tool. Data collectors and supervisors had trained for 1 day regarding the information to be collected and how to collect relevant information. Appraisal of the collected data was conducted daily by the principal investigator and supervisor for completeness. Charts with incomplete data during data collection were excluded.
Data processing and analysis
After data collection, data were checked via paper-wise observation, entered into Epi-data manager V.4.6, and exported to STATA V.14 for cleaning, edition, coding, and analysis. Exploratory analysis was performed before data description to determine the normality nature and presence of outliers. Then, categorical data were described using frequency tables and percentages, and continuous data were described using median with IQR and minimum and maximum values since the data were not normally distributed. Kaplan-Meier’s curve was constructed to estimate median time to attrition during the follow-up period and log-rank tests to compare survival curves among different categories of predictor variables. A life table was constructed to estimate the cumulative probability of attrition at different time intervals.
The univariate analysis was performed using Cox proportional hazard regression to identify the association between attrition and each independent variable in which variables with p≤0.25 were included in the multivariable analysis, to identify independent predictors of attrition. Multicolinearity was checked using the variance inflation factor (VIF) (mean VIF=1.36). Moreover, proportional hazard assumptions were checked using the Schoenfeld residual test (p> χ2=0.852). Harrell’s C was also computed (C=0.8359), which indicates that this study can correctly order survival times for pairs of children 83.6% of the time based on observations of fitted variables in the model. The Cox regression model for its fitness to the data was checked using the Cox-Snell residuals. Generally, we could conclude that the model fits successfully. In the final Cox proportional hazard model, statistical significance was declared at p<0.05, and the presence and strength of associations were summarised using an adjusted HR (AHR) with 95% CIs. Finally, texts, tables and graphs were used to present the study findings.
Patient and public involvement
It is not applicable for this study since patients were not directly involved. The study was conducted via patient chart review without contacting patients.
Results
From January 2013 to December 2019, an overall 288 (166 charts from Dilla Referral Hospital, 89 from Yirga Chefe Hospital and 33 from Bule Hospital) children less than 15 years started ART follow-up. Of those, 254 children under 15 years who fulfilled the inclusion criteria were reviewed, and 34 charts were excluded from the analysis.
Sociodemographic characteristics of HIV/AIDS-infected children
The finding revealed that among 137 (54%) male participants, 46 (33.6%) interrupted ART visits, whereas 50 (41.32%) of the 121 (47.6%) study participants living in rural areas interrupted their follow-up. The median age of the study participants at the initiation of ART follow-up was 6 years. About 129 (50.8%) children started ART follow-up at the age of 4–8 years. Among those children who lost from ART follow-up, 76.1% started follow-up below 9 years of age. One hundred eighty-six (73.2%) respondents were living with their parents. Of whom, 67 (36.0%) did not return to ART visits. Similarly, 21 (34.4%) of 61 (20.1%) children who lost either one or both parents failed to retain ART care. The study also showed that 171 (67.3%) caregivers of children were married, of which 58 (33.92%) children discontinued their follow-up (table 1).
Table 1.
Distribution of sociodemographic characteristics among HIV/AIDS-infected children who had follow-up at ART centres of Gedeo public hospitals, Southern Ethiopia, 2020 (N=254)
| Covariates | Category | Outcome status | Total (%) | |
| Attrition (%) | Censored (%) | |||
| Child age | ≤3 years | 24 (48) | 26 (52) | 50 (19.7) |
| 3–9 years | 46 (35.7) | 83 (64.3) | 129 (50.8) | |
| ≥9 years | 22 (29.3) | 53 (70.7) | 75 (29.5) | |
| Sex | Female | 46 (39.3) | 71 (60.7) | 117 (46.1) |
| Male | 46 (33.6) | 91 (66.4) | 137 (53.9) | |
| Religion of the caregiver | Orthodox | 31 (33) | 63 (67) | 94 (37.0) |
| Muslim | 12 (28.6) | 30 (71.4) | 42 (16.5) | |
| Protestant | 46 (41.4) | 65 (58.6) | 111 (43.7) | |
| Catholic | 3 (42.9) | 4 (57.1) | 7 (2.8) | |
| Residence | Rural | 50 (41.3) | 71 (58.7) | 121 (47.6) |
| Urban | 42 (31.6) | 91 (68.4) | 133 (52.4) | |
| Caregiver’s marital status | Single | 10 (41.7) | 14 (58.3) | 24 (9.4) |
| Married | 58 (33.9) | 113 (66.1) | 171 (67.3) | |
| Divorced | 8 (42.1) | 11 (57.9) | 19 (7.5) | |
| Widowed | 16 (40) | 24 (60) | 40 (15.8) | |
| Parent status | Both alive | 71 (36.8) | 122 (63.2) | 193 (76) |
| Either died | 19 (37.3) | 32 (62.7) | 51 (20.1) | |
| Both died | 2 (20) | 8 (80) | 10 (3.9) | |
| Caregiver’s HIV status | Reactive | 60 (35.9) | 107 (64.1) | 167 (65.8) |
| Non-reactive | 32 (36.8) | 55 (63.2) | 87 (34.2) | |
| Occupation of caregiver | Governmental employee | 3 (18.7) | 13 (81.3) | 16 (6.30) |
| Housewife | 46 (38.3) | 74 (61.7) | 120 (47.24) | |
| Self-employee | 41 (38) | 67 (62) | 108 (42.52) | |
| Others | 2 (20) | 8 (80) | 10 (3.94) | |
| Educational status of the caregiver | Illiterate | 45 (34.6) | 85 (65.4) | 130 (51.2) |
| Read and write | 18 (40.9) | 26 (59.1) | 44 (17.3) | |
| Grade 1–8 | 21 (43.8) | 27 (56.2) | 48 (18.9) | |
| Grade 9–12 | 3 (27.3) | 8 (72.7) | 11 (4.3) | |
| College and above | 5 (23.8) | 16 (76.2) | 21 (8.3) | |
ART, antiretroviral treatment.
Clinical and disease-related characteristics of HIV/AIDS-infected children
The median CD4+cell count at baseline was 606 cells/µL. From the overall 254 subjects, more than two-thirds of study participants (69.3%) had baseline CD4 cell count greater than 350 cells/µL, of which 45 (25.6%) experienced attrition while 9% of those who had CD4+ counts of ≤200 cells/µL attrited from ART follow-up. Among those children who started ART with an advanced clinical stage (WHO III or IV), 15.4% interrupted ART follow-up. Furthermore, 51 (68.9%) of the 74 (29.1%) children who had haemoglobin <10 mg/dL at the initial HIV/AIDS diagnosis were found to be absent sometime after initiation of ART follow-up. Among those children below the age of 5 years (102), 13 (12.8%) had delayed developmental status at baseline. Fifty-five (21.7%) respondents developed an opportunistic infection, of which 25 (45.5%) had diarrhoea and 12 (21.8%) had pneumonia. Almost 50% of participants who had tuberculosis coinfection discontinued their follow-up. Only 87 (34.3%) children knew their serostatus at ART follow-up initiation. Among children who started ART follow-up with non-disclosed serostatus, 33 (36.7%) had discontinued ART visits (table 2).
Table 2.
Clinical and disease-related characteristics of HIV/AIDS-infected children who had follow-up at ART centres of Gedeo public hospitals, Southern Ethiopia, 2020 (N=254)
| Covariates | Category | Outcome status | Total (%) | |
| Attrition (%) | Censored (%) | |||
| Developmental status (<5 years) | Appropriate | 32 (36) | 57 (64) | 89 (87.2) |
| Delayed | 10 (76.9) | 3 (23.1) | 13 (12.8) | |
| Functional status (>5 years) | Working | 25 (29.8) | 59 (70.2) | 84 (55.3) |
| Ambulatory | 18 (30.5) | 41 (69.5) | 59 (38.8) | |
| Bedridden | 7 (77.8) | 2 (22.2) | 9 (5.9) | |
| WFA | Z-score >−2 | 72 (32.1) | 152 (67.9) | 224 (88.2) |
| Z-score ≤−2 | 20 (66.7) | 10 (33.3) | 30 (11.8) | |
| HFA | Z-score >−2 | 75 (32.8) | 154 (67.2) | 229 (90.2) |
| Z-score ≤−2 | 17 (68) | 8 (32) | 25 (9.8) | |
| Baseline haemoglobin | <10 mg/dL | 51 (68.9) | 23 (31.1) | 74 (29.1) |
| ≥10 mg/dL | 41 (22.8) | 139 (77.2) | 180 (70.9) | |
| Baseline CD4+ count | ≤200 | 23 (65.7) | 12 (34.3) | 35 (13.8) |
| >200–350 | 24 (55.8) | 19 (44.2) | 43 (16.9) | |
| ≥350 | 45 (25.6) | 131 (74.4) | 176 (69.3) | |
| WHO clinical stage | I and II | 53 (30.5) | 121 (69.5) | 174 (68.5) |
| III and IV | 39 (48.8) | 41 (51.2) | 80 (31.5) | |
| Disclosure status | Disclosed | 52 (34.4) | 99 (65.6) | 151 (59.45) |
| Not disclosed | 40 (38.8) | 63 (61.2) | 103 (40.55) | |
| Opportunistic infections | No | 66 (33.2) | 133 (66.8) | 199 (78.35) |
| Yes | 26 (47.3) | 29 (52.7) | 55 (21.65) | |
| Tuberculosis | No | 82 (35.2) | 151 (64.8) | 233 (91.7) |
| Yes | 10 (47.6) | 11 (52.4) | 21 (8.3) | |
ART, antiretroviral treatment; HFA, height-for-age; WFA, weight-for-age.
Treatment-related characteristics of HIV/AIDS-infected children
Fifty-nine (23.3%) participants had poor or fair adherence. Among those, 47 (79.7%) lost from follow-up. Of the total 254 participants, 32 (12.6%) children had not received cotrimoxazole preventive therapy (CPT), and 17 (53.1%) of them had attrited from follow-up. Regarding the baseline ART regimen, 195 (76.8%), 39 (15.4%) and 20 (7.9%) study subjects started with nevirapine-based (NVP), efavirenz-based and other regimens correspondingly. The majority (83.7%) of children who initiated ART with the NVP-based regimen had failed to retain care. Moreover, 78 (30.7%) respondents suffered drug side effects while 74 (29.1%) children changed their initial ART regimen. More than half (56.4%) of children who experienced drug side effects had exhausted attending ART visits (table 3).
Table 3.
Distribution of treatment-related characteristics among HIV/AIDS-infected children who had follow-up at ART centres of Gedeo public hospitals, Southern Ethiopia, 2020 (N=254)
| Covariates | Category | Outcome status | ||
| Attrition (%) | Censored (%) | Total (%) | ||
| Baseline ART regimen | D4T-3TC-NVP | 14 (42.4) | 19 (57.6) | 33 (12.99) |
| AZT-3TC-NVP | 56 (37.1) | 95 (62.9) | 151 (59.45) | |
| TDF-3TC-EFV | 3 (20) | 12 (80) | 15 (5.91) | |
| ABC-3TC-EFV | 2 (28.6) | 5 (71.4) | 7 (2.76) | |
| AZT-3TC-EFV | 7 (41.2) | 10 (58.8) | 17 (6.69) | |
| ABC-3TC-NVP | 7 (63.6) | 4 (36.4) | 11 (4.33) | |
| Others | 3 (15) | 17 (85) | 20 (7.87) | |
| Adherence | Good | 45 (23.1) | 150 (76.9) | 195 (76.77) |
| Fair | 4 (66.7) | 2 (33.3) | 6 (2.36) | |
| Poor | 43 (81.1) | 10 (18.9) | 53 (20.87) | |
| CPT | Given | 75 (33.8) | 147 (66.2) | 222 (87.4) |
| Not given | 17 (53.1) | 15 (46.9) | 32 (12.6) | |
| Drug side effect | Yes | 44 (56.4) | 34 (43.6) | 78 (30.7) |
| No | 48 (27.3) | 128 (72.7) | 176 (69.3) | |
| Drug substitution | Yes | 22 (29.7) | 52 (70.3) | 74 (29.1) |
| No | 70 (38.9) | 110 (61.1) | 180 (70.9) | |
| Last outcome | Active/on follow-up | 0 (0) | 127 (100) | 127 (50.0) |
| Transfer out | 0 (0) | 35 (100) | 35 (13.8) | |
| Attrition/interrupted | 92 (100) | 0 (0) | 92 (36.2) | |
ABC, abacavir; ART, antiretroviral treatment; AZT, zidovudine; CPT, cotrimoxazole preventive therapy; D4T, stavudine; EFV, efavirenz; NVP, nevirapine; 3TC, lamivudine; TDF, tenofovir.
Comparison of survival status using Kaplan-Meier and log-rank test
The Kaplan-Meier failure curve increased stepwise as the follow-up time increased, and it crossed the survival function at the survival probability of 0.5 (figure 2).
Figure 2.
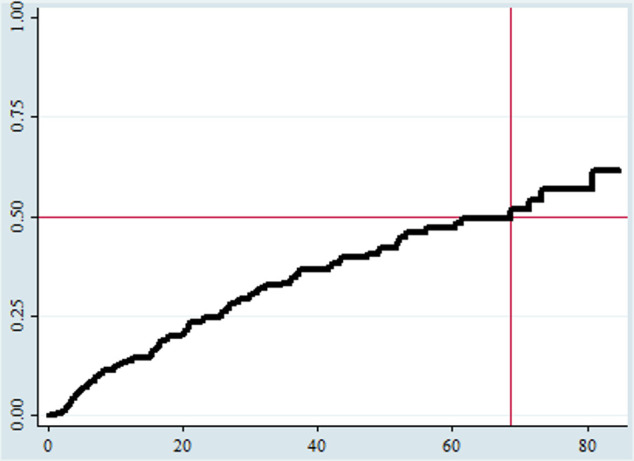
An overall Kaplan-Meier failure estimate among HIV/AIDS-infected children who had ART follow-up at ART clinics of Gedeo public hospitals, Southern Ethiopia, 2020 (N=254). The Y-axis represents the probability of failure, whereas the X-axis indicates the analysis time in months. ART, antiretroviral treatment.
Survival function and incidence of attrition
We have retrospectively followed children for a minimum of 29 days and a maximum of 84 months with a median follow-up of 26.4 months. Ninety-two (36.2%, N=254) children had attrited from ART follow-up, of which 70 (76.1%, n=92) were lost to follow-up and 22 (23.9%, n=92) children died. The rest, 162 (64.8%) participants, were considered censored. Among those, 127 (78.4%) were active on follow-up; and the rest, 35 (21.6%) cases, had transferred out to other health facilities. Fifty-seven (48%) of attrition cases occurred after 60 months of the follow-up.
The total child-month observation was 8145.33 child-months or 678.775 child-years with an incidence attrition rate of 11.3 (95% CI: 9.21 to 13.86) per 1000 child-month observations or 13.5 (95% CI: 11 to 16.6) per 100 child-year observations. The median survival time was 68.73 months. The cumulative probabilities of attrition were 0.14, 0.35, 0.47, and 0.62 at the end of 12, 36, 60, and 84 months of follow-up in that order.
Predictors of attrition among children who were on ART
In the univariate analysis, variables such as child age, residence, parent’s marital status, WFA, HFA, developmental milestone, baseline haemoglobin, baseline WHO clinical stage, baseline CD4 count, opportunistic infection, presence of tuberculosis, CPT, adherence to ART, ART drug side effect and drug substitution were found to have a p value less than 0.25 and included in the multivariable analysis. On the other hand, variables that contain less than 20% events per cell, such as parents’ status, occupation, parents’ education and functional status, were excluded from the final model. Likewise, the duration of ART follow-up violated the proportional hazard assumptions and thus further excluded from the multivariable analysis.
Finally, Cox proportional hazard model (multivariable analysis) showed that being underweight, baseline low CD4 count, suboptimal ART adherence, delayed developmental stage at follow-up initiation and a haemoglobin level of <10 mg/dL were significantly associated with attrition at 5% level of significance (table 4).
Table 4.
Cox proportional hazard regression analysis output for predictors of attrition among HIV/AIDS-infected children in Gedeo public hospitals, Southern Ethiopia, 2020 (N=254)
| Covariates | Category | Outcome status | P>|z| | Crude HR (95% CI) | P>|z| | Adjusted HR (95% CI) | |
| Attrited | Censored | ||||||
| Child age | 92 | 162 | 0.205 | 0.96 (0.90 to 1.02) | 0.131 | 0.82 (0.63 to 1.06) | |
| Residence | Rural | 50 | 71 | 0.088 | 1.43 (0.95 to 2.16) | 0.348 | 1.50 (0.64 to 3.47) |
| Urban | 42 | 91 | — | — | — | — | |
| Caregiver’s marital status | Single | 10 | 14 | 0.204 | 1.55 (0.80 to 3.03) | 0.730 | 1.29 (0.31 to 5.38) |
| Married | 58 | 113 | 0.287 | 1.49 (0.71 to 3.13) | 0.399 | 1.71 (0.49 to 5.99) | |
| Divorced | 8 | 11 | 0.569 | 1.17 (0.67 to 2.05) | 0.380 | 1.62 (0.55 to 4.75) | |
| Widowed | 16 | 24 | — | — | — | — | |
| Developmental status (for <5 years) | Appropriate | 32 | 57 | — | — | — | — |
| Delayed | 10 | 3 | 0.002 | 3.15 (1.53 to 6.49) | 0.021* | 3.60 (1.22 to 10.66) | |
| WFA | Normal | 72 | 152 | — | — | — | — |
| Underweight | 20 | 10 | <0.001 | 3.28 (1.98 to 5.45) | 0.007* | 5.91 (1.61 to 21.71) | |
| HFA | Normal | 75 | 154 | — | — | — | — |
| Stunted | 17 | 8 | <0.001 | 2.88 (1.69 to 4.89) | 0.215 | 0.39 (0.09 to 1.74) | |
| Baseline haemoglobin | <10 mg/dL | 51 | 23 | <0.001 | 3.87 (2.56 to 5.85) | 0.005* | 3.12 (1.41 to 6.93) |
| ≥10 mg/dL | 41 | 139 | — | — | — | — | |
| Baseline CD4+ count | ≤200 | 23 | 12 | <0.001 | 2.53 (1.57 to 4.06) | 0.004* | 4.43 (1.62 to 12.18) |
| >200 | 69 | 150 | — | — | — | — | |
| WHO stage | Early | 53 | 121 | — | — | — | — |
| Advanced | 39 | 41 | 0.015 | 1.67 (1.11 to 2.53) | 0.068 | 2.29 (0.94 to 5.59) | |
| OIs | No | 66 | 133 | — | — | — | — |
| Yes | 26 | 29 | 0.161 | 1.38 (0.88 to 2.18) | 0.395 | 1.44 (0.62 to 3.40) | |
| Presence of TB | No | 82 | 151 | — | — | — | — |
| Yes | 10 | 11 | 0.148 | 1.63 (0.84 to 3.15) | 0.240 | 0.51 (0.17 to 1.57) | |
| Adherence | Good | 45 | 150 | — | — | — | — |
| Suboptimal | 47 | 2 | <0.001 | 5.55 (3.65 to 8.46) | 0.004* | 3.45 (1.50 to 7.94) | |
| CPT | Given | 75 | 147 | — | — | — | — |
| Not given | 17 | 15 | 0.019 | 1.88 (1.11 to 3.18) | 0.290 | 1.81 (0.60 to 5.40) | |
| ARV drug side effect | Yes | 44 | 34 | 0.009 | 1.73 (1.15 to 2.61) | 0.125 | 0.51 (0.21 to 1.21) |
| No | 48 | 128 | — | — | — | — | |
| ARV drug substitution | Yes | 22 | 52 | — | — | — | — |
| No | 70 | 110 | 0.007 | 1.96 (1.20 to 3.19) | 0.153 | 1.90 (0.79 to 4.61) | |
*Significant at 5% level of significance.
ARV, antiretroviral drugs; CPT, cotrimoxazole preventive therapy; HFA, height-for-age; OIs, opportunistic infections; TB, tuberculosis; WFA, weight-for-age.
Those children having low haemoglobin levels (<10 mg/dL) were 3.12 times (AHR=3.12; 95% CI: 1.41 to 6.93) at an increased hazard of attrition than their counterparts. Children with delayed developmental milestones had a 3.6 increased risk of attrition (AHR=3.60; 95% CI: 1.22 to 10.66) when compared with those with an appropriate developmental milestone. Likewise, underweight children at baseline had a sixfold increased hazard of follow-up interruption than those having ideal weight (WFA z-score ≥−2) (AHR=5.91; 95% CI: 1.61 to 21.71). Furthermore, children who had CD4 count ≤200 cells/µL at the beginning of ART follow-up were 4.43 times (AHR=4.43; 95% CI: 1.62 to 12.18) at an increased hazard of follow-up interruption compared with their counterparts. Moreover, patients who had suboptimal adherence at baseline were three and a half times at a higher risk of experiencing attrition compared with those who had good adherence (AHR=3.45; 95% CI: 1.50 to 7.94).
Discussion
This study aimed to determine the incidence and predictors of attrition among HIV/AIDS-infected children on ART follow-up. In this study, the overall incidence rate of attrition was 13.5 per 100 child-year observations (95% CI: 11 to 16.6). Moreover, decreased baseline haemoglobin level, low baseline CD4 level, poor/fair ART adherence, delayed developmental stage at baseline and being underweight were found to predict children’s attrition from follow-up.
The current study showed that the overall incidence of attrition was 13.5 per 100 child-years of observation (95% CI: 11 to 16.6) which was higher than the findings of previous studies conducted in Thailand,28 Myanmar,3 Asian Pacific countries29 and Addis Ababa.21 This discrepancy could be due to the variation in access and quality of healthcare services among those countries. The level of awareness towards healthcare-seeking behaviour among those populations may vary as well. The length of follow-up would also contribute to the variation in the incidence rate where the previous study21 used only 2 years of follow-up data compared with 7 years in this study.
On the contrary, higher figures were reported from studies conducted in West Africa,14 Uganda,30 sub-Saharan Africa31 and East African countries.32 The possible explanation could be the emergence of more potent and palatable ART drugs these days. Moreover, the current guideline recommends frequent visits with enhanced adherence support and family involvement in ART care.6 26 The other possible reason might be the difference in sample size since previous studies conducted on larger sample sizes, N=17 155 in East African study32 and N=2170 in West African study.14
According to the study result, children who had low haemoglobin levels (<10 mg/dL) were 3.12 times at an increased hazard of attrition than their counterparts. This finding was in agreement with reports of other studies.3 11 21 33–35 The possible justification could be anaemia is associated with reduced immune defence resulting in decreased tolerance and absorption of ART drugs. Consequently, this may worsen the disease progression and retarding the response to standard therapy, and eventually, end up with exhaustion. Besides, some ART regimens were associated with anaemia that could further exacerbate the pre-existing condition.26 Another explanation could be decreased ART tolerance because of decreased absorption and the effect of immune defence from anaemia. To strengthen this evidence, about two-thirds (66.1%) of participants in our study started with zidovudine-containing regimen, of that 38.1% of children had experienced attrition.
This study also showed that children who had CD4 count ≤200 cells/µL at the beginning of ART follow-up were 4.43 times at an increased hazard of follow-up interruption than their counterparts did. The findings of previous studies conducted in Asian and African countries were consistent with the current study result.3 11 14 32 33 36–38 The explanation could be because immune suppression could expose children to further associated infections (opportunistic infections), contribute to rapid viral replication and HIV/AIDS progression, and hinder the desired therapeutic effect. As a result, the above mechanisms will result in exhaustion and non-compliance to ART follow-up leading to missed follow-up.
Moreover, HIV/AIDS-infected children who had suboptimal adherence at baseline were three and a half times at an increased hazard to experience attrition compared with those who had good adherence to ART drugs at initiation (AHR=3.45; 95% CI: 1.50 to 7.94). No statistically significant association was reported by earlier studies except the Addis Ababa study.21 The rationale could be suboptimal adherence to ART regimens contributes to increased viral replication and poor immunological and clinical outcomes. Suboptimal adherence would also be associated with an increased risk of ART drug resistance. Consequently, HIV/AIDS-infected children might become hopeless and exhausted to follow their ART visits. On the other hand, children are dependent on others to attend their ART follow-up visits and to take medications timely and in recommended dosages. Thus, caretaker-related issues might also influence adherence. Additionally, children frequently encountered adherence issues for several reasons.6 26 27
Likewise, children who were underweight at baseline had sixfold increased hazard of follow-up interruption than those patients having ideal weight (WFA z-score ≥−2). This finding was in line with the study done in Myanmar,3 Bali,35 Côte d’Ivoire,34 Uganda30 and a combined report in East Africa.33 The possible justification could be that undernutrition would lead to several undesired health effects, including compromised immunity, decreased effectiveness and response to ART drugs, and the emergence of complications and new coinfections. These in turn aid the further HIV replication and disease progression so that patients might prefer to interrupt their follow-up or lead to death.6 39
The other significantly associated factor in this study was a delayed developmental milestone. Children with delayed developmental milestones had a 3.6 increased risk of attrition when compared with those with an appropriate developmental milestone. Children are dependent on their parents/caregivers to accomplish their needs and responsibilities, including attending follow-up visits and timely intake and dosage of ART drugs. It could be like adding fire on the chaff when there is a developmental delay. In resource-limited countries like Ethiopia, it is challenging to assign regular caregivers to attend and fulfil the requirements of children in such circumstances. Moreover, the developmental delay may also be associated with diminished immune capability, further complicating the immunological and clinical recovery, thus affecting retention at care.
Although this study reported pertinent findings by considering censored observations for analysis and used longer follow-up period to estimate cumulative incidence of attrition, it has some limitations. First, the study involves patient chart review so that the effects of some important variables such as laboratory findings (eg, viral load) were left unevaluated. Second, excluding charts absent during the data collection period and those charts with missing data may underestimate or overestimate the study findings.
Conclusion
Retention to ART care is challenging in the paediatric population with such a high attrition rate. The incidence of attrition was found to be high in this study. Immune suppression (CD4+ count <200), anaemia (haemoglobin <10 mg/dL), underweight (WFA z-score <−2), delayed developmental milestone and ART non-adherence were independent predictors of attrition from ART care. Hence, it is crucial to detect and control the identified predictors promptly. Serious adherence support and strengthened nutritional provision with monitoring strategies are also essential.
Supplementary Material
Acknowledgments
The authors would like to thank data collectors, supervisors, hospital staff, and administrators for their unreserved efforts and commitment. The authors would also like to appreciate Dilla University, for covering the data collection costs, and Addis Ababa University, for chasing this chance.
Footnotes
Contributors: All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, data acquisition, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal, to which this work has been submitted; and agreed to be accountable for all aspects of the work.
Funding: KBB has gotten financial support from Dilla University to cover the perdiem of data collectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Additional data that support the findings of this study are available from the corresponding author upon reasonable request and can be shared upon legal request via bayayibignabez@gmail.com.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The Addis Ababa University College of Health Sciences Institutional Review Board (IRB) offered the ethical approval (protocol number: 02/20/SNM) and an official cooperation letter written to respective hospitals from the school of nursing and midwifery. The IRB waived such that the research could be done by record review without contacting patients since the study was retrospective (conducted via patient chart review). Permission was obtained from selected hospitals on behalf of patients as well as ART clinics of each hospital. Data coding and aggregate reporting were used to ensure anonymity and confidentiality, and the questionnaires were locked.
References
- 1.Edmonds A, Yotebieng M, Lusiama J, et al. The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: a cohort study. PLoS Med 2011;8:e1001044. 10.1371/journal.pmed.1001044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HIV/AIDS JUNPo . UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS, 2013: 201. [Google Scholar]
- 3.Minn AC, Kyaw NTT, Aung TK, et al. Attrition among HIV positive children enrolled under integrated HIV care programme in Myanmar: 12 years cohort analysis. Glob Health Action 2018;11:1510593. 10.1080/16549716.2018.1510593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulugeta A, Assefa H, Tewelde T. Determinants of survival among HIV positive children on antiretroviral therapy in public hospitals, Addis Ababa, Ethiopia. Qual Prim Care 2017;25:235–41. [Google Scholar]
- 5.Organization WH . Progress report 2016: prevent HIV, test and treat all: who support for country impact. World Health Organization, 2016. [Google Scholar]
- 6.Federal Minstry Of Health . National guidelines for comprehensive HIV prevention, care, and treatment. Addis Ababa, Ethiopia, 2017: 256. [Google Scholar]
- 7.WHO - UNICEF . Global Update on Hiv Treatment 2013 : Glob Updat HIV Treat 2013 results, impact Opportunities, 2013. [Google Scholar]
- 8.EDHS E. Demographic and health survey 2016: key indicators report. The DHS Program ICF 2016;363:364. [Google Scholar]
- 9.Harries AD, Zachariah R, Lawn SD, et al. Strategies to improve patient retention on antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health 2010;15 Suppl 1:70–5. 10.1111/j.1365-3156.2010.02506.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joint United Nations Programme on HIV/AIDS . Global HIV & AIDS statistics – 2018 fact sheet, 2018. [Google Scholar]
- 11.Abuogi LL, Smith C, McFarland EJ. Retention of HIV-infected children in the first 12 months of anti-retroviral therapy and predictors of attrition in resource limited settings: a systematic review. PLoS One 2016;11:e0156506. 10.1371/journal.pone.0156506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox MP, Rosen S. Systematic review of retention of pediatric patients on HIV treatment in low and middle-income countries 2008-2013. AIDS 2015;29:493–502. 10.1097/QAD.0000000000000559 [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Uria G. Description of the cascade of care and factors associated with attrition before and after initiating antiretroviral therapy of HIV infected children in a cohort study in India. PeerJ 2014;2:e304. 10.7717/peerj.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekouevi DK, Azondekon A, Dicko F, et al. 12-Month mortality and loss-to-program in antiretroviral-treated children: the IeDEA pediatric West African database to evaluate AIDS (pWADA), 2000-2008. BMC Public Health 2011;11:519. 10.1186/1471-2458-11-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melaku Z, Lulseged S, Wang C, et al. Outcomes among HIV-infected children initiating HIV care and antiretroviral treatment in Ethiopia. Trop Med Int Health 2017;22:474–84. 10.1111/tmi.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed I, Lemma S. Mortality among pediatric patients on HIV treatment in sub-Saharan African countries: a systematic review and meta-analysis. BMC Public Health 2019;19:149. 10.1186/s12889-019-6482-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNairy ML, Lamb MR, Carter RJ, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda, and Tanzania. J Acquir Immune Defic Syndr 2013;62:e70–81. 10.1097/QAI.0b013e318278bcb0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peacock-Villada E, Richardson BA, John-Stewart GC. Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics 2011;127:e423–41. 10.1542/peds.2009-2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organization WH . Retention in HIV programmes: defining the challenges and identifying solutions: meeting report, 13-15 September 2011, 2012. [Google Scholar]
- 20.Lafort Y, Couto A, Sunderbrink U, et al. Validity of reported retention in antiretroviral therapy after roll-out to peripheral facilities in Mozambique: results of a retrospective national cohort analysis. PLoS One 2018;13:e0198916. 10.1371/journal.pone.0198916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biru M, Hallström I, Lundqvist P, et al. Rates and predictors of attrition among children on antiretroviral therapy in Ethiopia: a prospective cohort study. PLoS One 2018;13:e0189777. 10.1371/journal.pone.0189777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucciardini R, Fragola V, Abegaz T, et al. Predictors of attrition from care at 2 years in a prospective cohort of HIV-infected adults in Tigray, Ethiopia. BMJ Glob Health 2017;2:e000325. 10.1136/bmjgh-2017-000325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eticha EM, Gemeda AB. Predictors of mortality among adult patients enrolled on antiretroviral therapy in Hiwotfana specialized university Hospital, eastern Ethiopia: retrospective cohort study. Journal of HIV for Clinical and Scientific Research 2018;5:007–11. [Google Scholar]
- 24.Gesesew HA, Mwanri L, Ward P, et al. Factors associated with discontinuation of anti-retroviral therapy among adults living with HIV/AIDS in Ethiopia: a systematic review protocol. JBI Database System Rev Implement Rep 2016;14:26–37. 10.11124/jbisrir-2016-2451 [DOI] [PubMed] [Google Scholar]
- 25.Mekuria LA, Prins JM, Yalew AW, et al. Retention in HIV care and predictors of attrition from care among HIV-infected adults receiving combination anti-retroviral therapy in Addis Ababa. PLoS One 2015;10:e0130649. 10.1371/journal.pone.0130649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach: who, 2016. [PubMed] [Google Scholar]
- 27.Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV . Guidelines for the use of antiretroviral agents in pediatric HIV infection. AIDS info, 2018. [Google Scholar]
- 28.Teeraananchai S, Kerr SJ, Puthanakit T, et al. Attrition and mortality of children receiving antiretroviral treatment through the universal coverage health program in Thailand. J Pediatr 2017;188:e1:210–6. 10.1016/j.jpeds.2017.05.035 [DOI] [PubMed] [Google Scholar]
- 29.Lo Y-R, Kato M, Phanuphak N, Ying-Ru L, et al. Challenges and potential barriers to the uptake of antiretroviral-based prevention in Asia and the Pacific region. Sex Health 2014;11:126–36. 10.1071/SH13094 [DOI] [PubMed] [Google Scholar]
- 30.Massavon W, Barlow-Mosha L, Mugenyi L, et al. Factors determining survival and retention among HIV-infected children and adolescents in a community home-based care and a Facility-Based Family-Centred approach in Kampala, Uganda: a cohort study. Isrn Aids 2014;2014:1–13. 10.1155/2014/852489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marazzi MC, De Luca S, Palombi L, et al. Predictors of adverse outcomes in HIV-1-infected children receiving combination antiretroviral treatment: results from a dream cohort in sub-Saharan Africa. Pediatr Infect Dis J 2014;33:295–300. 10.1097/INF.0b013e3182a0994b [DOI] [PubMed] [Google Scholar]
- 32.Leroy V, Malateste K, Rabie H, et al. Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. J Acquir Immune Defic Syndr 2013;62:208–19. 10.1097/QAI.0b013e31827b70bf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Farhat J, Schramm B, Nicolay N, et al. Mortality and clinical outcomes in children treated with antiretroviral therapy in four African vertical programmes during the first decade of paediatric HIV care, 2001-2010. Trop Med Int Health 2017;22:340–50. 10.1111/tmi.12830 [DOI] [PubMed] [Google Scholar]
- 34.Auld AF, Tuho MZ, Ekra KA, et al. Temporal trends in mortality and loss to follow-up among children enrolled in Côte d'Ivoire's national antiretroviral therapy program. Pediatr Infect Dis J 2014;33:1134–40. 10.1097/INF.0000000000000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefanie J, Sawitri A, et al. Predictors of loss to follow up and mortality among children ≤12 years receiving anti retroviral therapy during the first year at a referral hospital in Bali. Public Health and Preventive Medicine Archive 2016;4. 10.15562/phpma.v4i2.65 [DOI] [Google Scholar]
- 36.Ditekemena J, Luhata C, Bonane W, et al. Antiretroviral treatment program retention among HIV-infected children in the Democratic Republic of Congo. PLoS One 2014;9:e113877. 10.1371/journal.pone.0113877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tene G, Lahuerta M, Teasdale C, et al. High retention among HIV-infected children in Rwanda during scale-up and decentralization of HIV care and treatment programs, 2004 to 2010. Pediatr Infect Dis J 2013;32:e341–7. 10.1097/INF.0b013e31828c2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.KIDS-ART-LINC Collaboration . Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in sub-Saharan Africa. J Acquir Immune Defic Syndr 2008;49:523–31. 10.1097/QAI.0b013e31818aadce [DOI] [PubMed] [Google Scholar]
- 39.Takarinda KC, Harries AD, Shiraishi RW, et al. Gender-Related differences in outcomes and attrition on antiretroviral treatment among an HIV-infected patient cohort in Zimbabwe: 2007-2010. Int J Infect Dis 2015;30:98–105. 10.1016/j.ijid.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Additional data that support the findings of this study are available from the corresponding author upon reasonable request and can be shared upon legal request via bayayibignabez@gmail.com.