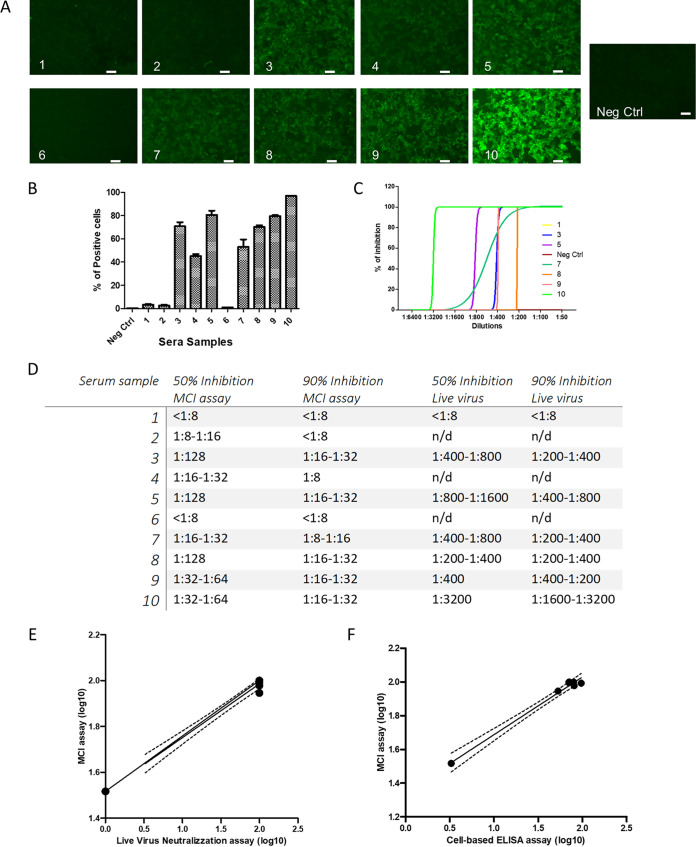
FIG 2.
Cell-based immunofluorescent (CBI) and MCI assays to assess SARS-CoV-2 convalescent-phase sera. (A and B) HEK293T cells transiently transfected with the plasmid encoding S were incubated with the indicated sera (diluted 1:20 at 4°C for 1 h, followed by incubation with protein G Alexafluor 488 (1:500) as secondary antibody. Selected fields are shown in panel A. The percentage of positive cells (average from three independent experiments ± SEM) is quantitated in panel B. (C) Selected sera (1, 3, 5, 7–10) were tested for neutralization activity against live SARS-CoV-2 virus. The graph shows the results of a single experiment representative of the three biological replicates. (D) Table comparing the dilutions for 50% and 90% inhibition in MCI and live virus assays for the selected sera. (E) Correlation between the MCI and live virus neutralization data showing serum samples 1, 3, 5, 7, 8, 9, and 10. (F) Comparison of MCI and cell-based ELISA results. Sera with the best binding and neutralization activity (samples 3, 5, and 7 to 10) and a negative control (sample 1) were used. The dotted lines represent the confidence intervals (95% CI).