Figure 1.
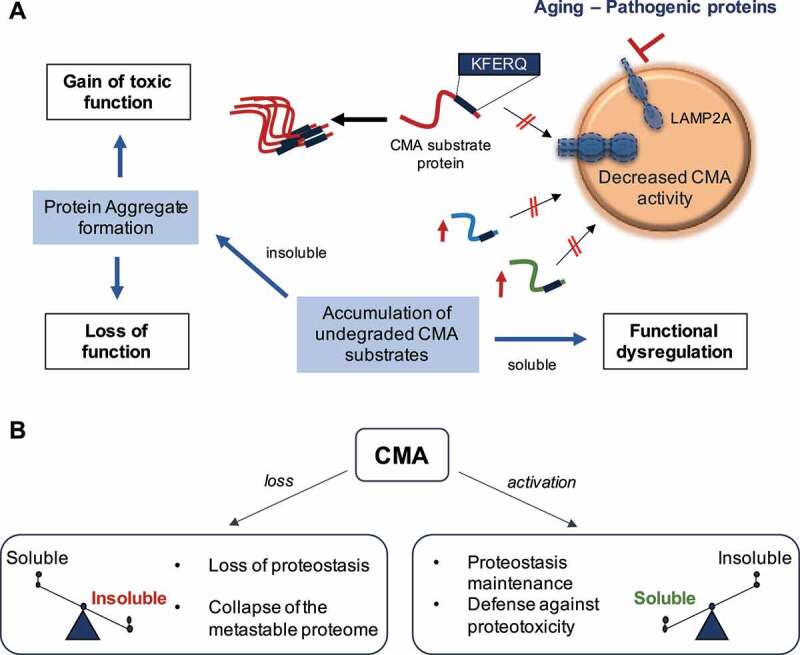
Contribution of chaperone-mediated autophagy (CMA) to neuronal protein homeostasis. (A) Aging and/or pathogenic proteins reduce CMA activity, which in turn lead to the accumulation of undegraded CMA substrates in the form of protein aggregates. Neuronal aggregates upon CMA blockage have two direct consequences: (1) a gain of toxic function due to the presence of the aggregate itself, and (2) a loss function due to the entrapment of functional proteins inside the aggregate and their subsequent loss of function. Furthermore, failure to timely degrade proteins by CMA to terminate their function can lead to dysregulation of other neuronal functions. (B) CMA loss leads to a switch of the proteome toward insolubility, whereas CMA activation, even in the context of preexisting neuropathology, restores proteostasis and contributes to the neuronal defense against proteotoxicity. LAMP2A: lysosomal-associated membrane protein 2A
