Table 3.
In vitro antiproliferative activity of compounds 10a–10j against human cancer cells.
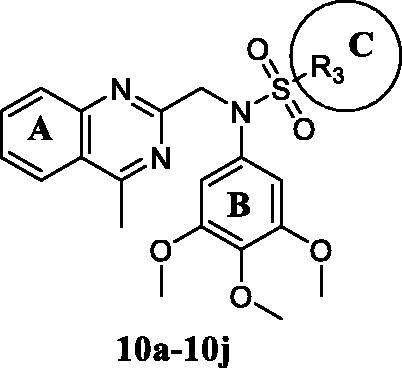
| |||||
|---|---|---|---|---|---|
| Compounds | R 3 | IC50 (μM)a |
|||
| MGC-803 | HCT-116 | PC-3 | MCF-7 | ||
| 10a | Phenyl | 6.62 ± 0.82 | 16.7 ± 1.03 | 28.2 ± 2.12 | >50 |
| 10b | 4-F-phenyl | 0.92 ± 1.07 | 3.84 ± 0.14 | 4.72 ± 1.02 | 5.46 ± 0.87 |
| 10c | 4-Br-phenyl | 0.82 ± 0.11 | 1.49 ± 0.22 | 1.81 ± 0.26 | 1.40 ± 0.25 |
| 10d | 2-Cl-phenyl | >50 | 18.7 ± 1.27 | >50 | 22.1 ± 0.31 |
| 10e | 4-CH3-phenyl | 1.00 ± 0.08 | 2.48 ± 0.12 | 4.01 ± 0.07 | 1.68 ± 0.12 |
| 10f | 2,4,6-(CH3)3-phenyl | >50 | >50 | >50 | >50 |
| 10g | 4-C(CH3)3-phenyl | 12.8 ± 1.38 | 22.4 ± 1.67 | 18.9 ± 1.57 | 28.1 ± 1.82 |
| 10h | 3,4-(OCH3)2-phenyl | 14.2 ± 1.03 | 22.9 ± 1.34 | 36.6 ± 0.34 | 18.5 ± 0.81 |
| 10i | 2-thienyl | 16.8 ± 1.42 | 20.4 ± 1.92 | 27.8 ± 2.02 | 30.2 ± 1.81 |
| 10j | 2-pyridyl | 14.7 ± 1.88 | 24.8 ± 2.13 | 28.2 ± 1.98 | 34.2 ± 1.83 |
| 5-Fu | – | 6.82 ± 1.12 | 14.4 ± 1.73 | 17.1 ± 1.42 | 12.1 ± 1.28 |
aIn vitro antiproliferative activity was assayed by exposure for 48 h.
