Figure 1.
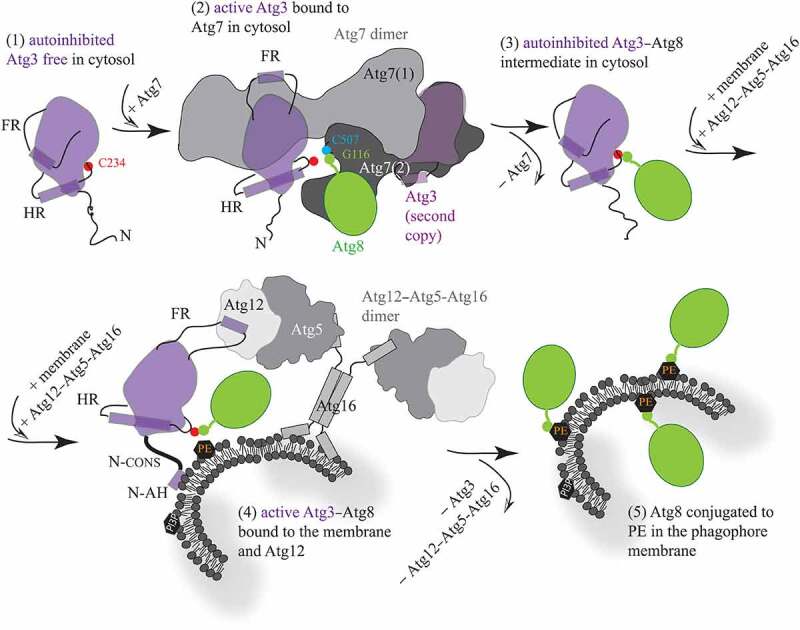
Schematic model for molecular mechanisms in Atg3. (1) Free, autoinhibited Atg3 becomes active upon the interaction of a short helical element in the flexible loop (FR) with the N-terminal domain of Atg7. (2) The flexible FR allosterically regulates enzyme activation that is manifested by helical extension in the handle region (HR) of Atg3 and, thereby, repositioning of the catalytic cysteine (C234). These movements allow for formation of a thioester bond between C234 and the C-terminal glycine (G116) in Atg8 that, after adenylation, resides in the activation domain of Atg7, and is covalently linked to the Atg7 catalytic cysteine (C507). (3) After the transfer of Atg8 from Atg7 to Atg3, the Atg7 dimer is released. The FR presumably reengages the Atg3 surface, causing autoinhibition of the covalent Atg3–Atg8 intermediate. (4) The cytosolic Atg3 molecule attaches to the membrane by embedding the N-terminal amphipathic helix (N-AH) into the lipid bilayer. This event is communicated via the N-terminal conserved region (N-CONS) to a structural rearrangement around C234. Membrane binding of Atg3 is either preceded or followed by binding of a short structural element in the FR to Atg12 in the Atg12–Atg5-Atg16 complex. The Atg3 FR–Atg12 interaction can reposition C234 via an allosteric mechanism, presumably similar to that in the Atg3–Atg7 complex. Structural changes induced by the membrane and Atg12 near Atg3 C234 facilitate the transfer of Atg8 G116 to PE. (5) After Atg3 and Atg12–Atg5-Atg16 detach from the assembly, Atg8 is lipidated on the phagophore membrane
