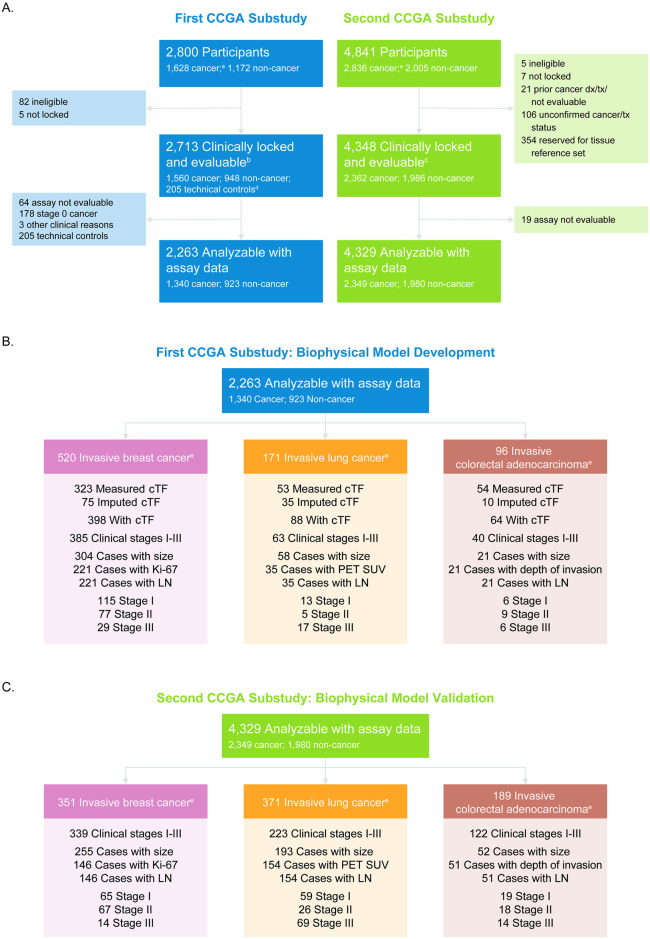
Fig 3. CONSORT diagram.
(A) CONSORT diagram depicting the number of clinically evaluable cases with evaluable assay results for model generation from the first CCGA substudy (left) and model validation from the second CCGA substudy (right). (B) Cases available for model development from the first CCGA substudy and (C) cases available for model validation from the second CCGA substudy for breast (left), lung (center), and colorectal cancers (right). Cases were filtered by availability of clinical data (size of primary tumor, Ki-67 for breast cancer, PET SUV for lung cancer, depth of microinvasion for colorectal cancer, respectively, and presence of tumor-involved lymph nodes) for modeling and available ground truth or imputed tumor fraction. cTF: Circulating Tumor fraction, LN: information on number of tumor-involved lymph nodes, PET SUV, positron emission tomography standardized uptake value. aAt enrollment, prior to confirmation of cancer status. bBy First CCGA substudy definition. cBy Second CCGA substudy definition. dNon-smoking participants under the age of 35. eConfirmed cancer status.