Fig. 7. Transport mechanism of PepT2.
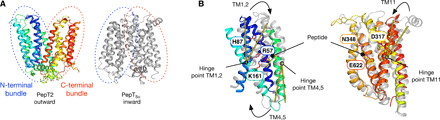
(A) Cartoon representation of PepT2 in the outward open state compared to PepTSo (PDB: 2XUT) in the inward open state. The N- and C-terminal six-helix bundles are marked with blue and red dotted lines, respectively. (B) Structural overlay of the N-terminal bundle (left) and C-terminal bundle (right) colored as in (A). The key structural changes in the N-terminal bundles are highlighted by yellow lines, showing the angles of movement in TM2, TM4, and TM11. Key side chains involved in peptide and proton binding are indicated (purple sticks) and labeled. The docked l-Ala-Phe peptide is shown for reference.
