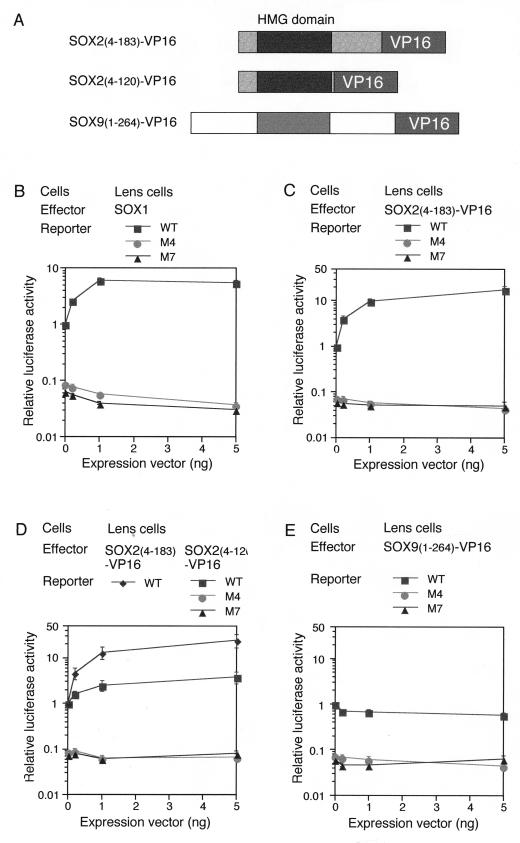
FIG. 4.
Fusion proteins of SOX HMG domains bearing the VP16 activation domain still require the partner factor for transactivation. (A) Schematic structures of SOX-VP16 fusion proteins. C-terminal domains of SOX2 and SOX9 carrying the transactivation potential were replaced with the VP16 activation domain. (B to E) Luciferase reporter plasmids were the same as in Fig. 1 and carried the WT, M4 (loss of SOX binding), or M7 (loss of δEF3 binding) form of the DC5 enhancer. Luciferase reporter plasmids were cotransfected with various amounts of effector vectors (0, 0.2, 1, and 5 ng) expressing either SOX1 (B), SOX2(4-183)-VP16 (C and D), SOX2(4-120)-VP16 (D), or SOX9(1-264)-VP16 (E) into lens cells. Luciferase activity generated by the reporter containing DC5-WT in the absence of exogenous SOX proteins was taken as 1 in each experiment. Luciferase expression of the reporters carrying the M4 or M7 mutation was ∼15-fold lower than that of the DC5-WT-carrying reporter. This finding indicates that in lens cells, the transfected reporter is already activated by endogenous SOX1/2/3. Therefore, the overall activation level of DC5 enhancer by SOX1/2/3 in lens cells is estimated to be 30- to 100-fold, and that by SOX2(4-183)-VP16 is ∼300-fold.