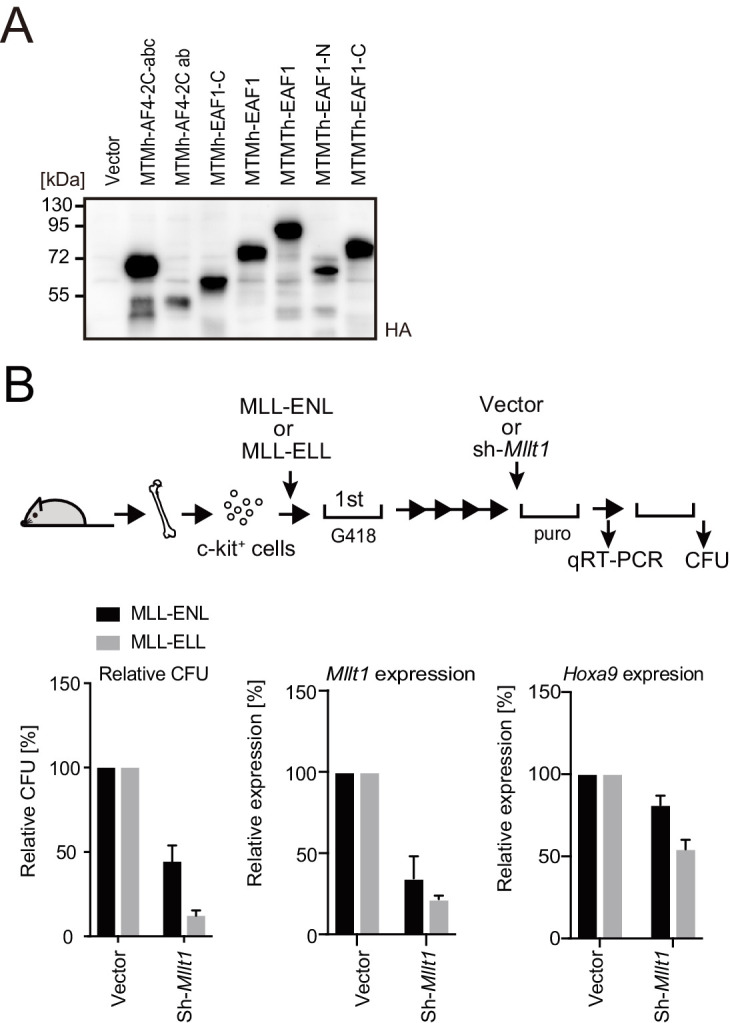
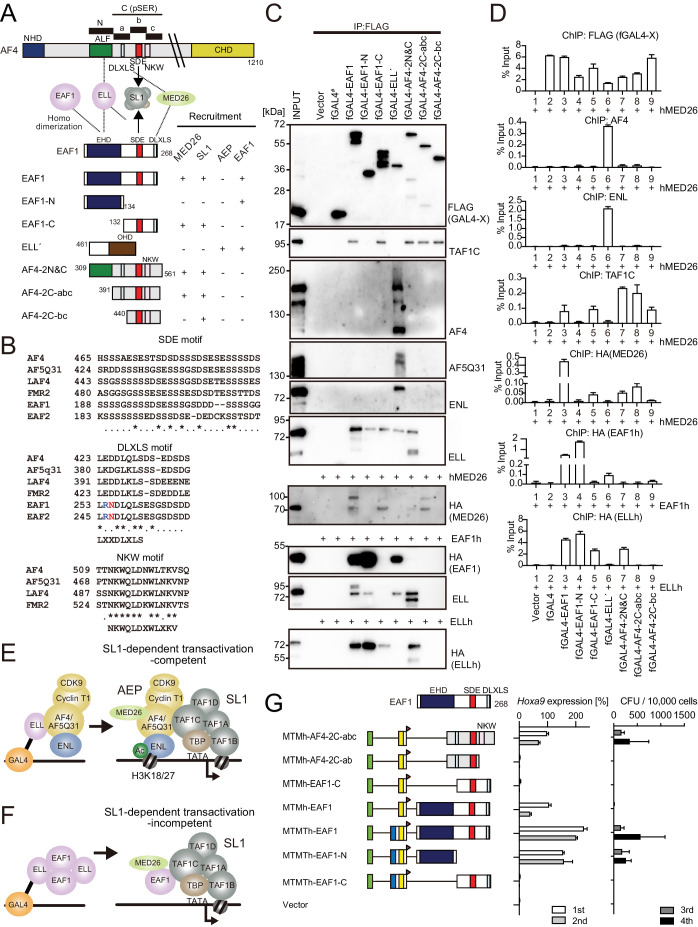
Figure 5. AF4 and EAF1 form distinct SL1/MED26-containing complexes.
(A) A schema of the structures of AF4, ELL, and EAF1. ALF is responsible for interaction with ELL, pSER is responsible for interaction with SL1, and DLXLS is responsible for interaction with MED26. EAF family homology domain (EHD) is responsible for homodimerization and interaction with ELL. NKW:NKW motif responsible for transcriptional activation. (B) Alignment of the amino acid sequences of the SDE, DLXLS, and NKW motifs of human AF4 family proteins and EAF family proteins. Conserved residues are indicated by asterisks. (C) Association of EAF1, ELL, and AF4 domains with various associating factors on chromatin. Immunoprecipitation (IP)-western blotting of the chromatin fraction of HEK293TL cells transiently expressing various FLAG-tagged GAL4 fusion proteins, with or without indicated HA-tagged constructs, was performed as in Figure 4D. Endogenous proteins were detected by specific antibodies for each protein, while exogenous proteins were detected by antibodies for FLAG or HA tag. (D) Recruitment of various transcriptional regulatory proteins by EAF1, ELL, and AF4 domains. Chromatin immunoprecipitation (ChIP)-qPCR analysis of HEK293TL cells transiently expressing various combinations of FLAG-tagged GAL4 fusion proteins along with indicated HA-tagged constructs was performed as in Figure 4C. (E) Putative complex recruitment mediated by ELL-AF4 interaction. (F) Putative complex recruitment mediated by ELL-EAF1 interaction. (G) Structural requirement of MTM-AF4 pSER fusion and MTMT-EAF1 fusion. Various MTMh/MTMTh constructs fused with EAF1 domains were examined for the transformation of myeloid progenitors as in Figure 1A.
Figure 5—figure supplement 1. Roles for AF4/ENL/P-TEFb (AEP) in MLL-ELL-mediated leukemic transformation, related to Figure 5.