Figure 6. MLL-ELL transforms hematopoietic progenitors via association with AF4/ENL/P-TEFb (AEP), but not with EAF1 or p53.
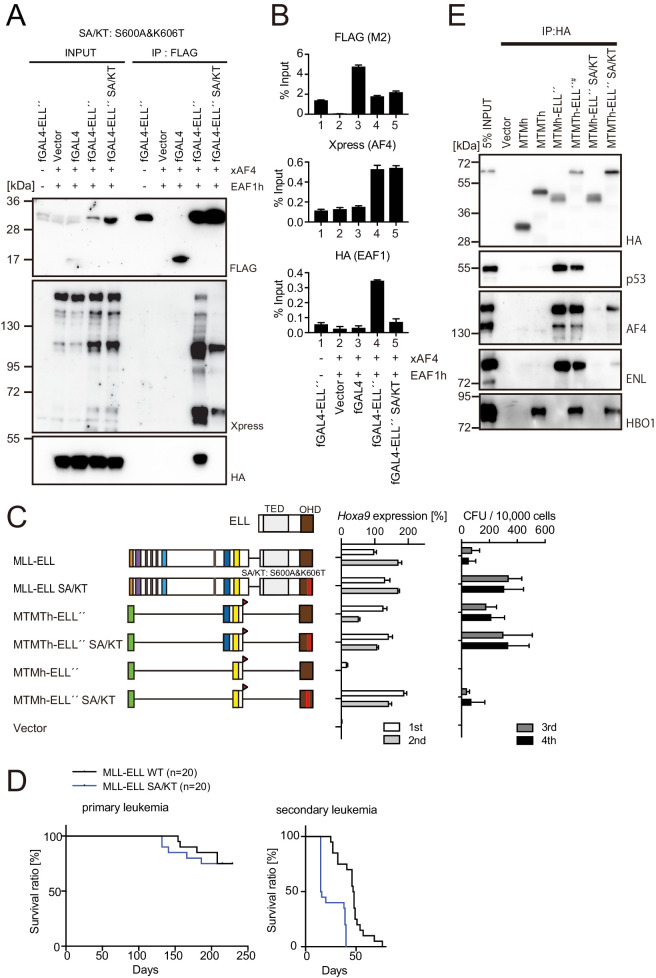
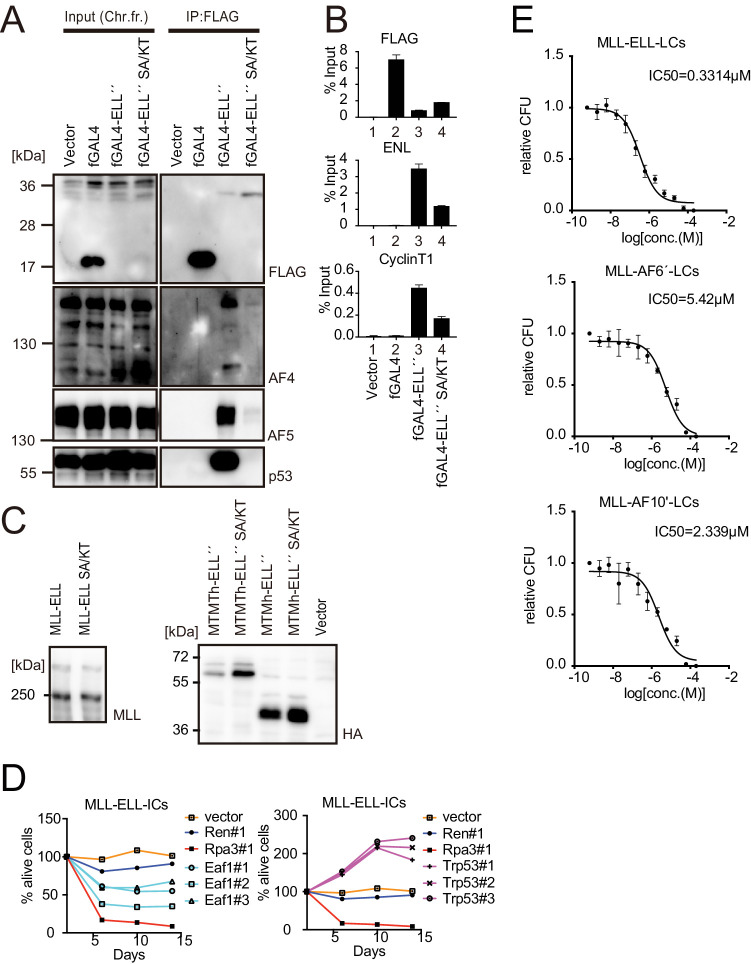
(A) Mutations of ELL selectively abrogate interaction with EAF1. Immunoprecipitation (IP)-western blotting of the chromatin fraction of HEK293TL cells transiently expressing FLAG-tagged GAL4-ELL proteins with or without S600A/K606T substitutions (SA/KT) along with xAF4 and EAF1h was performed as in Figure 4D. (B) Recruitment of exogenously expressed AF4 or EAF1 by ELL mutant proteins. Chromatin immunoprecipitation (ChIP)-qPCR analysis of HEK293TL cells transiently expressing FLAG-tagged GAL4-ELL proteins with or without the SA/KT mutation along with xAF4 and EAF1h was performed as in Figure 4C. (C) Effects of the SA/KT mutation on MLL-ELL-mediated leukemic transformation ex vivo. Various MLL-ELL constructs with or without the SA/KT mutation were examined for the transformation of myeloid progenitors as in Figure 1A. (D) Effects of the SA/KT mutation on MLL-ELL-mediated leukemic transformation in vivo. MLL-ELL or its SA/KT mutant was transduced to c-Kit-positive hematopoietic progenitors and transplanted into syngeneic mice (n = 20). Primary leukemia cells were harvested from the bone marrow and transplanted into secondary recipient mice (n = 20). (E) Enhanced interaction of ELL with AEP mediated by the trithorax homology domain 2 (THD2) domain. IP-western blotting of the chromatin fraction of virus-packaging cells, transiently expressing various HA-tagged MTMT-ELL fusion constructs (with or without the SA/KT mutation), was performed. Endogenous proteins co-purified with MLL-ELL proteins were visualized by specific antibodies.