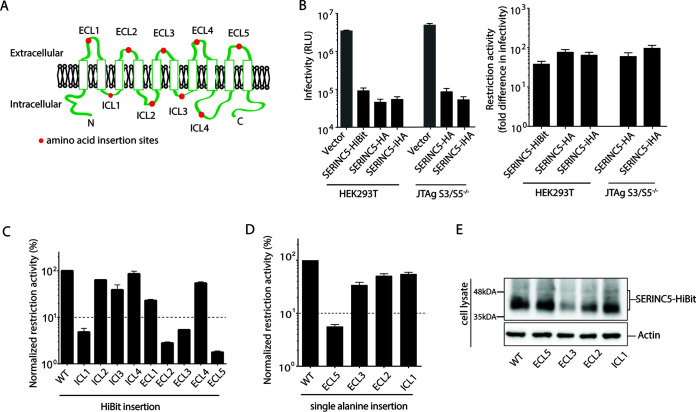
FIG 1.
Important role of extracellular loop 5 of SERINC5 in HIV-1 restriction. (A) Schematic illustration of SERINC5 based on TOPCON’s consensus prediction of transmembrane protein topology (39). Red dots represent the location of HiBit peptide or alanine insertions. (B) Infectivity of HIV-1 NL43ΔEnvΔNef pseudotyped with EnvNL produced in HEK293T and JTAg-SERINC−/− cells in the presence of vector only or various constructs of SERINC5 as indicated (left). SERINC5-HiBit and SERINC5-HA contained HiBit and HA peptide tags, respectively, at the C terminus of SERINC5. SERINC5-iHA contained an HA peptide tag located between residues 290 and 291 of SERINC5. Fold difference in infectivity in the absence and presence of SERINC5 constructs (as a measure of restriction activity of SERINC5 molecules) is shown (right). (C and D) Restriction activity of WT and a series of HiBit insertion mutants of SERINC5 (C) and a series of alanine insertion mutants of SERINC5 (D) against HIV-1 NL43ΔEnvΔNef pseudotyped with EnvNL that had been produced in HEK293T cells. The single-round infectivity was measured as β-galactosidase activity in TZM-bl target cells. The restriction activity was calculated as the fold difference of infectivity relative to the empty vector control, and the resultant values were normalized to SERINC5 WT, which was set to 100%. Mutants with normalized restriction activity of <10% (dotted lines) were considered to be significantly affected. (E) Steady-state cellular expression levels of the WT and indicated alanine-inserted mutants of SERINC5-HiBit that had been visualized with a Nano-Glo HiBit blotting system (Promega). Data are means and SD from 3 independent experiments.