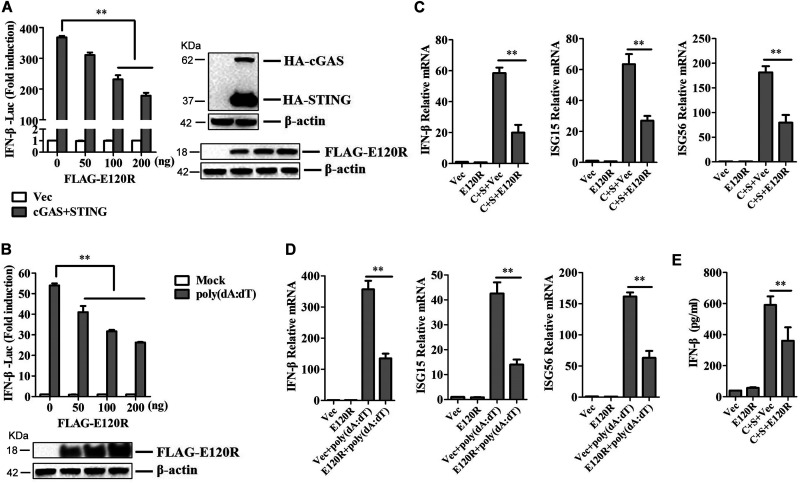
FIG 1.
E120R inhibited IFN-β production in HEK-293T cells. (A) HEK-293T cells were transfected with 0.1 μg/well of IFN-β-Luc expression plasmid and 0.01 μg/well of pRL-TK plasmid along with 0.1 μg/well of HA-cGAS and HA-STING expression plasmids, as well as increasing doses of FLAG-E120R expression plasmid (0, 50, 100, and 200 ng). At 24 hpt, the promoter activation of IFN-β was determined by using the Dual-Luciferase assay kit. Three independent experiments were performed with two technical replicates. (B) HEK-293T cells were transfected with 0.1 μg/well of IFN-β-Luc expression plasmid and 0.01 μg/well of pRL-TK plasmid along with increasing doses of FLAG-E120R expression plasmid (0, 50, 100, and 200 ng). At 24 hpt, cells were transfected with poly(dA·dT) (2 μg/ml) for 12 h. The promoter activation of IFN-β was evaluated through the Dual-Luciferase assay. Three independent experiments were performed with two technical replicates. (C) HEK-293T cells cultured in 6-well plates were transfected with 1 μg each of HA-cGAS and HA-STING expression plasmids and 1 μg each of FLAG vector or FLAG-E120R expression plasmid. At 24 hpt, the mRNA expression levels of IFN-β, ISG15, and ISG56 were determined by qPCR assay. Three independent experiments were performed with two technical replicates. C, cGAS; S, STING. (D) HEK-293T cells cultured in 6-well plates were transfected with 1 μg of FLAG vector or FLAG-E120R expression plasmid. At 24 hpt, cells were transfected with poly(dA·dT) (2 μg/ml) for 12 h, and the mRNA expression levels of IFN-β, ISG15, and ISG56 were measured by qPCR assay. Three independent experiments were performed with two technical replicates. (E) Transfection experiments were performed as described in the legend to panel C. The supernatant was collected, and the amounts of secreted IFN-β protein were measured using an ELISA kit. Three technical replicates were performed. Error bars show standard deviations. **, P < 0.01.