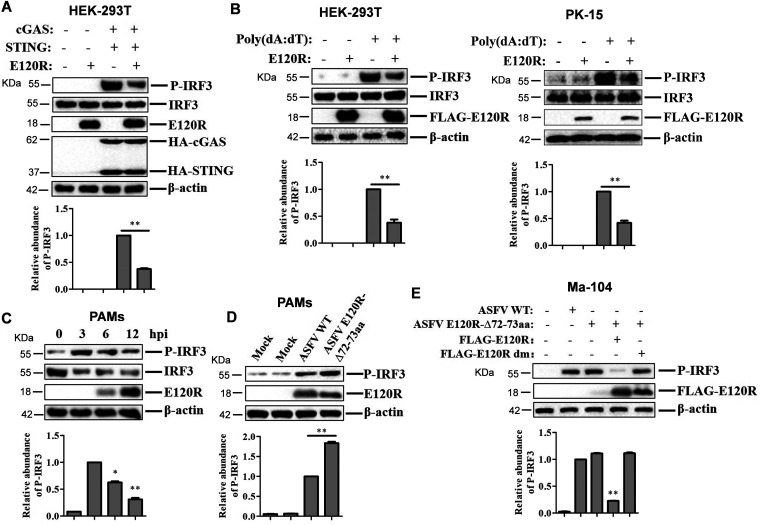
FIG 7.
E120R protein inhibited IRF3 phosphorylation. (A) HEK-293T cells were transfected with 2 μg of empty FLAG vector, 1 μg of HA vector, or 1 μg each of HA-cGAS and HA-STING expression plasmids and 2 μg of FLAG-E120R expression plasmid. At 24 hpt, the expression of IRF3, P-IRF3, E120R, cGAS, and STING proteins was detected by Western blotting. Three independent experiments were performed. (B) HEK-293T cells were transfected with 2 μg each of FLAG vector and/or FLAG-E120R expression plasmids. PK-15 cells were transfected with 1 μg each of FLAG vector and/or FLAG-E120R expression plasmids. At 24 hpt, the transfected cells were transfected with poly(dA·dT) (2 μg/ml) for 12 h. Expression of IRF3, P-IRF3, and E120R proteins was detected by Western blotting. Three independent experiments were performed. (C) PAMs were infected with ASFV (MOI of 2) for 0, 3, 6, or 12 h. Expression of IRF3, P-IRF3, and E120R proteins was detected by Western blotting. Three independent experiments were performed. (D) PAMs were mock infected or infected with ASFV WT or ASFV E120R-Δ72-73aa (MOI of 2) for 8 h. Expression of the P-IRF3 and E120R proteins was detected by Western blotting. Three independent experiments were performed. (E) Ma-104 cells cultured in 6-well plates were transfected with 4 μg of FLAG vector or FLAG-E120R or FLAG-E120R dm expression plasmid. At 24 hpt, the cells were infected with ASFV or ASFV E120R-Δ72-73aa (MOI of 2) for 4 h, and the levels of IRF3 phosphorylation were detected by Western blotting. Three independent experiments were performed. The change in abundance of P-IRF3 was determined by densitometric analysis using ImageJ Software and normalized to β-actin. Error bars show standard deviations. *, P < 0.05; **, P < 0.01.