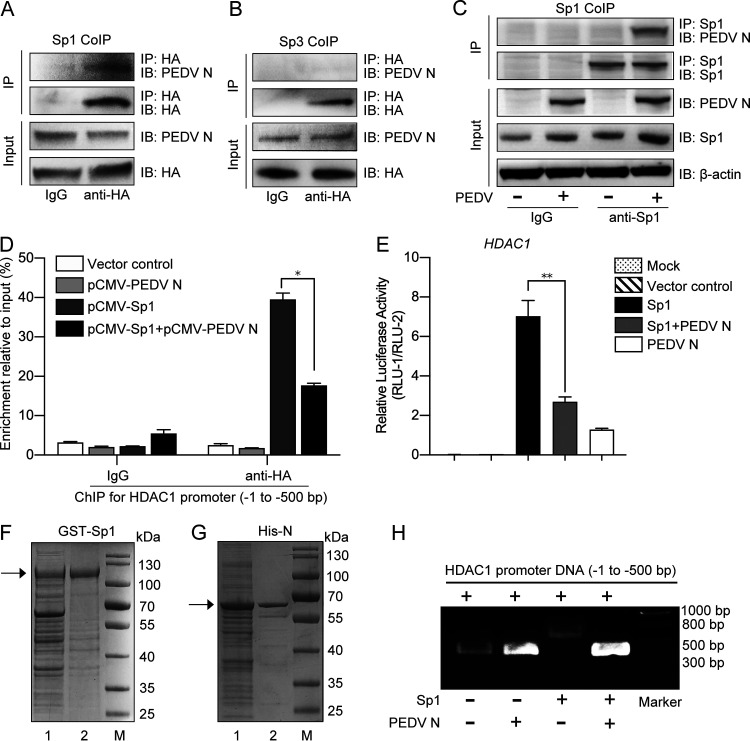
FIG 10.
Porcine epidemic diarrhea virus N protein interacted with Sp1 and inhibited its transcriptional activity. (A and B) Interaction of PEDV N protein with Sp1 or Sp3 in the HEK293T cells cotransfected with recombinant plasmids pCMV-N-Flag and pCMV-Sp1-HA or -Sp3-HA. The total cell lysates were immunoprecipitated with HA antibody, followed by immunoblotting with PEDV N monoclonal antibody. Total cell lysates immunoprecipitated with normal mouse IgG were used as controls. (C) Interaction of PEDV N protein with Sp1 in the PEDV-infected IPEC-J2 cells. The cell lysates immunoprecipitated by Sp1 antibody were immunoblotted with PEDV N antibody. Uninfected cell lysates were used as a control. (D) PEDV N protein affected Sp1 binding to the putative HDAC1 promoter (bp −1 to −500), as shown by ChIP assay. (E) PEDV N protein inhibited Sp1 transcriptional activity on the HDAC promoter in the HEK293T cells transfected with different combinations of pCMV-Sp1 and/or pCMV-N together with pGL3-Enhancer-PHDAC1 and pRL-TK. (F and G) Expression and purification of GST-Sp1 and His-N protein detected by SDS-PAGE: lane 1, total proteins after induction with IPTG; lane 2, recombinant proteins purified by affinity chromatography with a GST-binding resin or a His-binding resin. (H) PEDV N protein inhibited Sp1 binding to the HDAC promoter, as shown by gel shift assay. Migration of the target DNA was detected by 0.8% agarose gel electrophoresis.