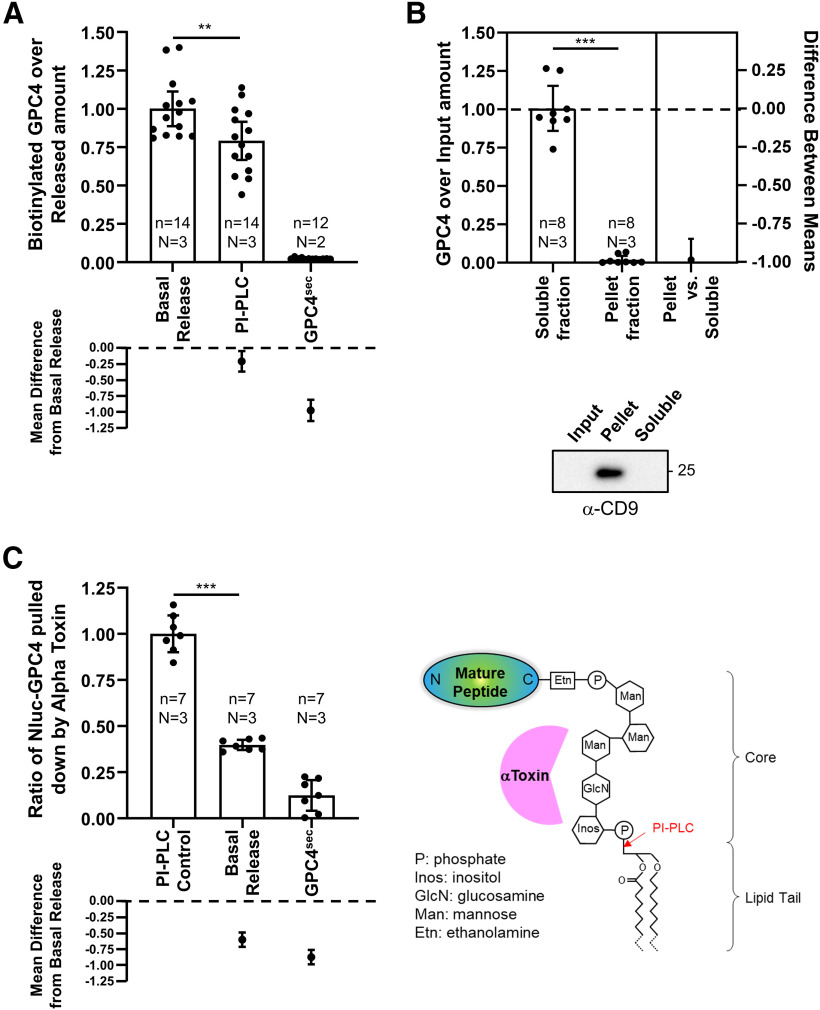
Figure 3.
Characterizing the release mechanism of GPC4 using Nluc-GPC4. A, After biotinylating the surface proteins of astrocytes expressing Nluc-GPC4, cells were further incubated in the fresh medium for 5 h. The released Nluc-GPC4 is collected from the medium, and the ratio of biotinylation of the released Nluc-GPC4 was measured (luciferase activity of avidin pulldown over medium input). As a control for the release from the cell surface, PI-PLC were treated during the incubation time. Both basal and PI-PLC induced GPC4 release occur from the cell surface. GPC4sec, which does not contain the GPI-anchor signal peptide and is thus not GPI-anchored, is directly secreted without membrane attachment and lacks biotinylation (basal vs PI-PLC ANOVA p = 0.0079, Cohen’s d = 1.012). B, Ultracentrifugation of ACMs containing Nluc-GPC4 was used to test for GPC4 association with extracellular vesicles as a potential release mechanism. Supernatant (soluble) fraction and pellet (vesicular) fraction Nluc were normalized to input Nluc luminescence. Nluc-GPC4 did not associate with the pellet (vesicular) fraction. An exosome marker, CD9, was enriched in the pellet (vesicular) fraction (t test p < 0.0001, Cohen’s d = 7.829). C, alpha toxin (AT), which binds to the glycan core of GPI-anchors, was used to pull down GPI-anchored proteins released through lipase activity. PI-PLC, lipase released Nluc-GPC4 is used as a positive control, while GPC4sec, lacking a GPI anchor, is used as a negative control. Nluc-GPC4 pulled down by AT is normalized to input Nluc-GPC4 measurements. Basal release shows partial binding to AT, indicating that ∼30% of Nluc-GPC4 is released through a lipase mechanism (PI-PLC vs basal release ANOVA p < 0.0001, Cohen’s d = 7.659); **p < 0.01, ***p < 0.001.