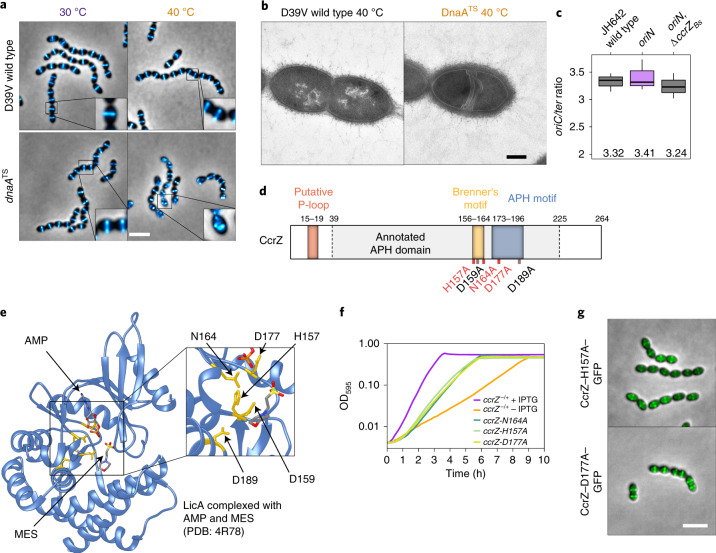
Fig. 5. CcrZ activates DnaA-dependent replication initiation.
a, Localization of FtsZ–mTurquoise2 in a thermosensitive DnaA strain (dnaATS) at permissive (30 °C) and non-permissive (40 °C) temperatures shows that dnaA inactivation leads to a similar phenotype as ccrZ inactivation. Scale bar, 3 µm. b, TEM of DnaATS at a non-permissive temperature (40 °C) indicates the presence of multiple septa, similar to a ΔccrZ mutant. Scale bar, 250 nm. c, When replication is driven in a RepN-dependent manner in B. subtilis (oriN), no decrease in ori/ter ratio can be observed in the absence of ccrZBs (oriN, ΔccrZBs). Mean values are indicated under the boxes. Data are shown as box (25th to 75th percentile) and whisker (1.5× IQR) plots; n = 3 independent samples. d, Schematic representation of CcrZ motifs. CcrZ has one putative domain annotated APH (phosphotransferase enzyme family; PFAM01636). Sequence alignment with several kinases revealed the presence of a conserved P-loop, APH and Brenner’s motifs, found in most phosphotransferases. Locations of mutations made for three essential (red) and two non-essential (black) conserved residues are shown underneath. e, LicA choline kinase structure complexed with AMP and MES buffer. The five residues indicated in yellow are conserved between CcrZ and LicA (and highly conserved within Firmicutes). f, Mutation of three of these five conserved residues in the putative ATP-binding pocket leads to growth defects. g, Localization of CcrZ–H157A–GFP and CcrZ–D177A–GFP is not impaired. Scale bar, 3 µm.