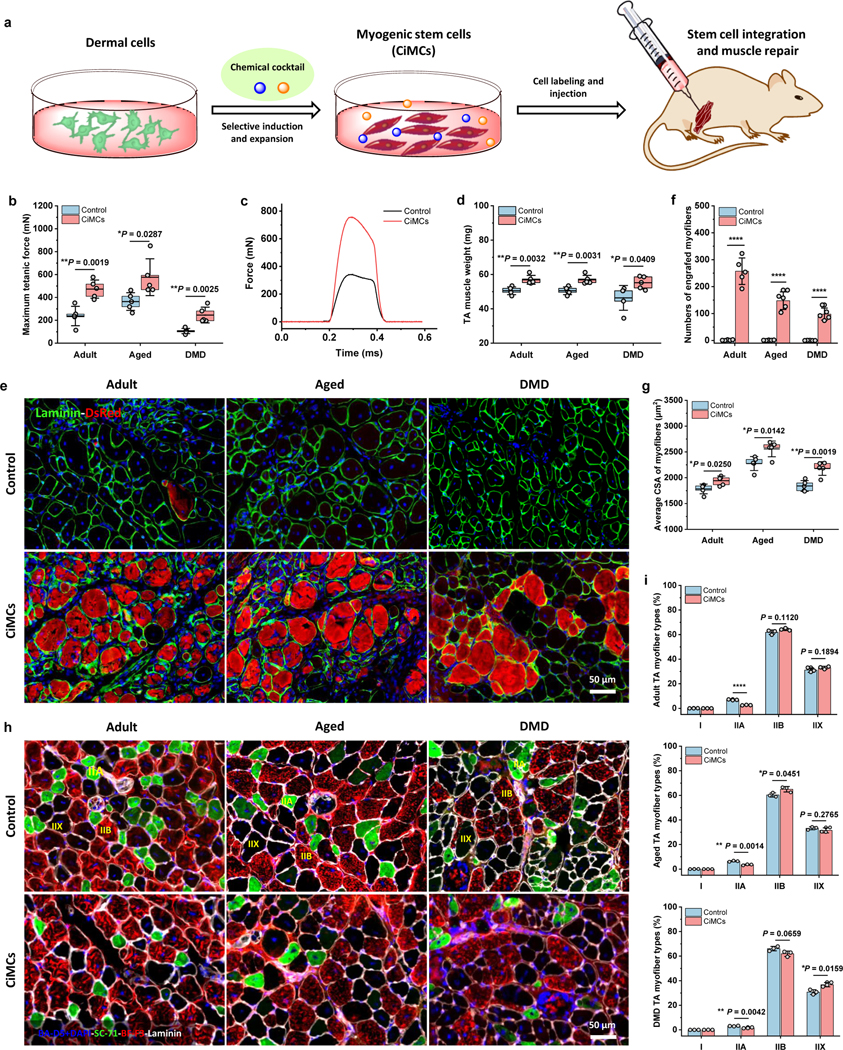
Fig. 6 |. In vivo engraftment of CiMCs promotes muscle regeneration.
a, Schematic of the injection of in vitro chemical-induced dermal cell-derived myogenic stem cells for muscle repair. b, Maximum isometric tetanic force of CTX-injured TA muscles in adult, aged and mdx mice after transplantation of dermal cells (negative control) or CiMCs at 4 weeks (n = 5 mice per group). c, Representative isometric tetanic force curves of control and CiMC-treated aged TA muscle at 4 weeks. d, Muscle wet weight of control and CiMC-treated adult, aged and mdx TA muscles at 4 weeks (n = 5 mice per group). e, DsRed-labeled control and CiMCs were transplanted into CTX-injured adult, aged and mdx TA muscles for 4 weeks. f, The number of DsRed myofibers in muscle tissue from (e) (n = 5 mice per group). g, Average CSA of centrally nucleated myofibers in adult, aged and mdx TA muscles transplanted with either control cells or CiMCs for 4 weeks (n = 5 mice per group). h, Representative myofiber (types I, IIA, and IIB) staining of the adult, aged and mdx TA muscles transplanted with either control cells or CiMCs for 4 weeks (n = 5 mice per group, n = 5 fields of view per group). i, The percentage of distinct myofiber types in (h) (n = 5 mice per group). Data are presented as mean ± SD. The box charts in (b,d,e,g) extend from the 25th to 75th percentiles and the line in the middle of the box is plotted at the mean value. Two-tailed Student’s t-test. *P < 0.05, **P < 0.01 and ****P < 0.0001.