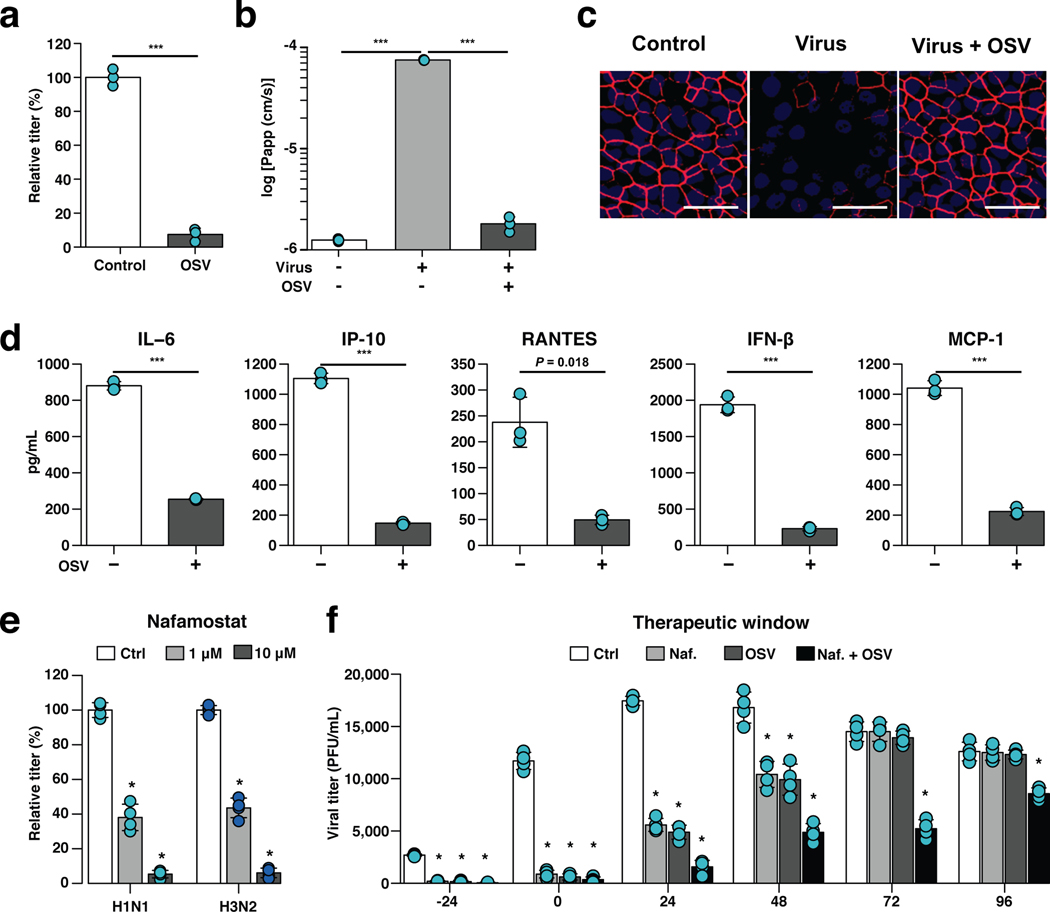
Fig. 3 |. Effects of anti-influenza therapeutics in the human Airway Chip.
a, Graph showing relative plaque titers of progeny virus in the absence (Ctrl) or presence of 1 μM oseltamivir acid (OSV) 48 h post-infection with WSN (H1N1; MOI = 0.1). b, Barrier permeability (log Papp) measured under control conditions within the human Airway Chip (- Virus) or 48 h post-infection with WSN (+ Virus) with (+) or without (−) OSV. c, Immunofluorescence micrographs showing the distribution of ZO1-containing tight junctions in airway epithelium under baseline conditions (Ctrl) or infected with WSN alone (Virus) or in the presence of OSV (Virus + OSV) 48 h post-infection (bar, 50 μm). d, Production of cytokines in human Airway Chip 48 h post-infection with WSN in the presence (+) or absence (−) of OSV. e, Virus titer detection showing the effects of Nafamostat at 1 μM (grey bars) or 10 μM (white bars) dose on virus replication of H1N1 and H3N2 in Airway chips 48 h post-infection compared to untreated chips (Ctrl, black bars). In (a-e), drugs were added at the same time that the chips were infected with virus. f, The effects of Nafamostat, oseltamivir and their combination on viral titers when added to H1N1 virus-infected human Airway Chips at indicated times (i.e., 24 hours before virus addition versus at the time as infection or 24, 48, 72, or 96 hours after infection). Effects on viral load were analyzed at 48 h following addition of drug. Note the synergistic effects of these two drugs at later times. Data represent mean ± s.d.; n = 4 biological chip replicates for e, f, and n = 3 for a, b, and d; ***, p < 0.001 in a,b,d and *, < 0.001 in e,f.