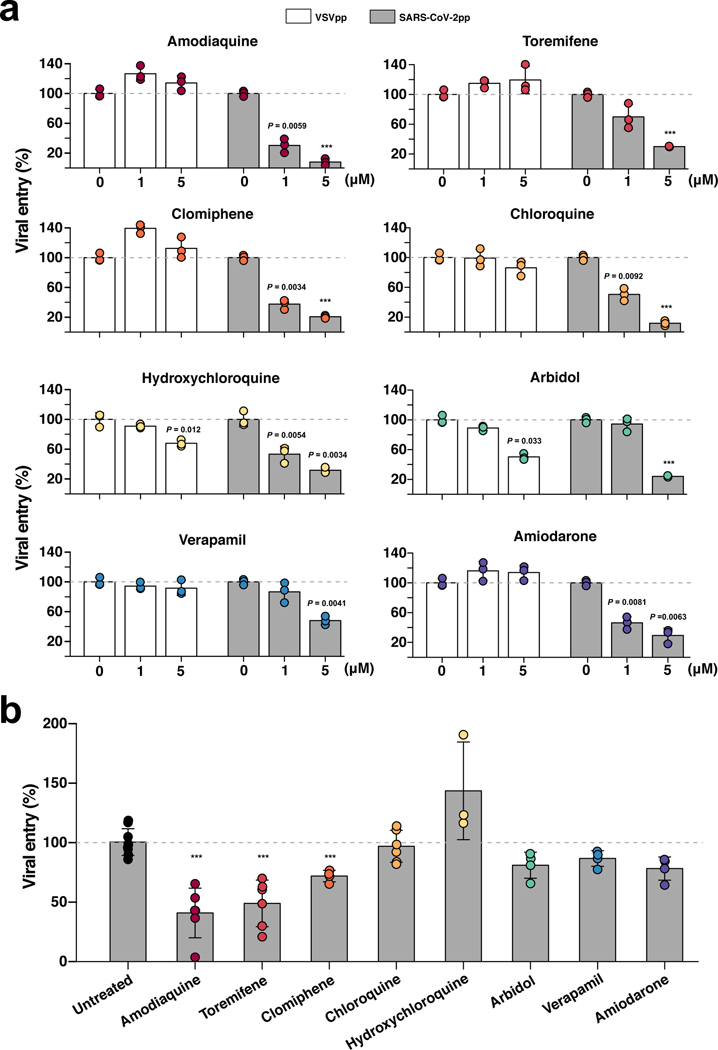
Fig. 4 |. Effects of FDA-approved drugs on pseudotyped SARS-CoV-2 viral entry in Huh-7 cells versus human Airway Chips.
a, Graphs showing the inhibitory effects of amodiaquine, toremifene, clomiphene, chloroquine, hydroxychloroquine, arbidol, verapamil, and amiodarone when added at 0, 1, or 5 μM to Huh-7 cells infected with SARS-CoV-2pp for 72 h (grey bars). The number of pseudoparticles in the infected cells was quantified by measuring luciferase activity; viral entry in untreated cells was set as 100%. VSVpp were tested in parallel to exclude toxic and nonspecific effects of the drugs tested (white bars). b, The efficacy of the same drugs in human Airway Chips infected with SARS-CoV-2pp. Amodiaquine, toremifene, clomiphene, chloroquine, hydroxychloroquine, arbidol, verapamil, and amiodarone were delivered into apical and basal channels of the chip at their respective Cmax in human blood, and one day later chips were infected with SARS-CoV-2pp while in the continued presence of the drugs for 2 more days. The epithelium from the chips were collected for detection of viral pol gene by qRT-PCR; viral entry in untreated chips was set as 100%. Data represent mean ± s.d.; n = 3 biological replicates for a, and n = 3–6 for b. ***, p < 0.001.