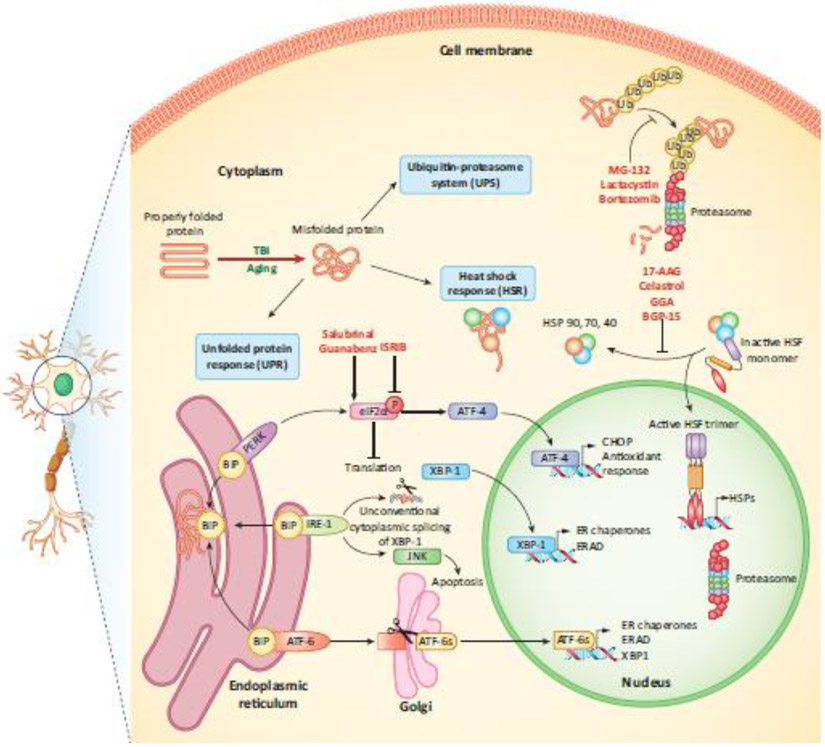
Figure 1 (Key Figure). Stress response pathways dysregulated with age and injury:
Three different stress pathways triggered by misfolded protein in neuronal and glial cells are illustrated – the heat shock response (HSR), the unfolded protein response (UPR), and the ubiquitin proteasome system (UPS). Therapeutic modulators of TBI are shown in red, indicating the pathways they affect. UPR: Misfolded proteins in the ER bind the stress-sensing chaperone, BiP, sequestering it away from PERK, IRE-1 and ATF-6 and activating them. PERK phosphorylates eIF2α leading to global repression of translation and increasing selective translation of ATF-4. IRE-1 triggers the unconventional cytoplasmic splicing of XBP-1, as well as activation of the JNK pathway. ATF-6 translocates to the Golgi apparatus and is processed to form the active transcription factor ATF-6s. Together, these mediators dictate the induction of pathways that attempt to restore normal cellular function by refolding or degrading misfolded proteins, and in the event of prolonged stress, activating apoptotic pathways. UPS: Misfolded proteins are tagged with a polyubiquitin chain and targeted to the cytoplasmic or nuclear proteasome for degradation. HSR: HSPs bind HSF and sequester it in an inactive monomeric form. During cellular stress, HSPs bind misfolded proteins, allowing HSF to trimerize, translocate to the nucleus and trigger the transcription of more HSPs. Abbreviations: BiP, Ig binding protein; PERK, PKR-like ER kinase; IRE-1, inositol requiring enzyme-1; ATF, activating transcription factor; eIF2α, eukaryotic initiation factor 2α; CHOP, C/EBP homologous protein; XBP-1, X-box binding protein-1; ER, endoplasmic reticulum; ERAD, ER associated degradation; JNK, Jun N-terminal kinase; Ub, ubiquitin; HSP, heat shock protein; HSF, heat shock factor.