Figure 1. Robust cargo recruitment to synthetic condensates via protein-protein interaction domains.
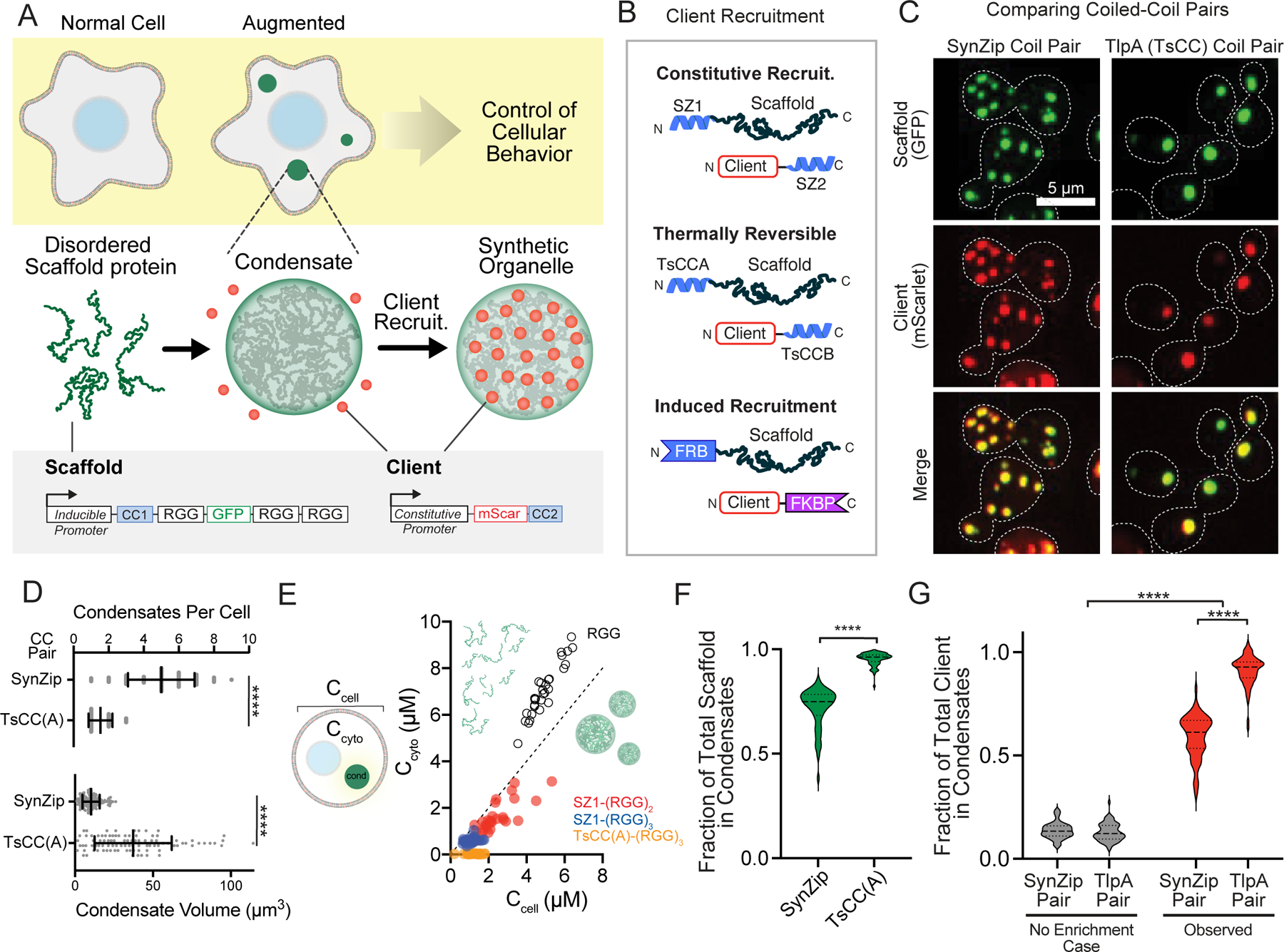
a, Schematic overview assembling synthetic organelles from disordered scaffold proteins to target clients and predictably modulate cellular functions. Scaffold: triple RGG, GFP tag, and a high affinity coiled-coil (CC) tag under the control of an inducible promoter. Client: fluorophore with a cognate CC under the control of a constitutive promoter. b, Client recruitment strategies: Synzips, thermally responsive coiled-coils, and rapamycin-induced dimerization domains. c, Representative images of yeast cell expressing a scaffold and client with cognate Synzip (left) or thermally responsive (right) CC pairs. Merged images show strong recruitment of client to condensates. d, Comparison of mean condensate number (top) and volume (bottom) for each scaffold type n = 60 cells for SynZip and n = 75 cells for TsCC(A) scaffolds. Error bars, s.d. Significance calculated by unpaired, two-tailed t-test (****, p ≤ 0.0001). e, Steady-state cytoplasmic concentration of scaffold outside of condensates (Ccyto) as a function of total cellular concentration (Ccell) for 30 cells per scaffold type. Dashed line, slope of 1. f, Violin plots of fraction of total scaffold protein present in condensates for cells as in d. Significance calculated by unpaired, two-tailed t-test (****, p ≤ 0.0001). g, Violin plots of fraction of total client recruitment to condensates with each CC pair compared to expected percentage for condensates of same size without recruitment in cells as in d. Significance calculated by one-way ANOVA (****, p ≤ 0.0001).
