Figure 2.
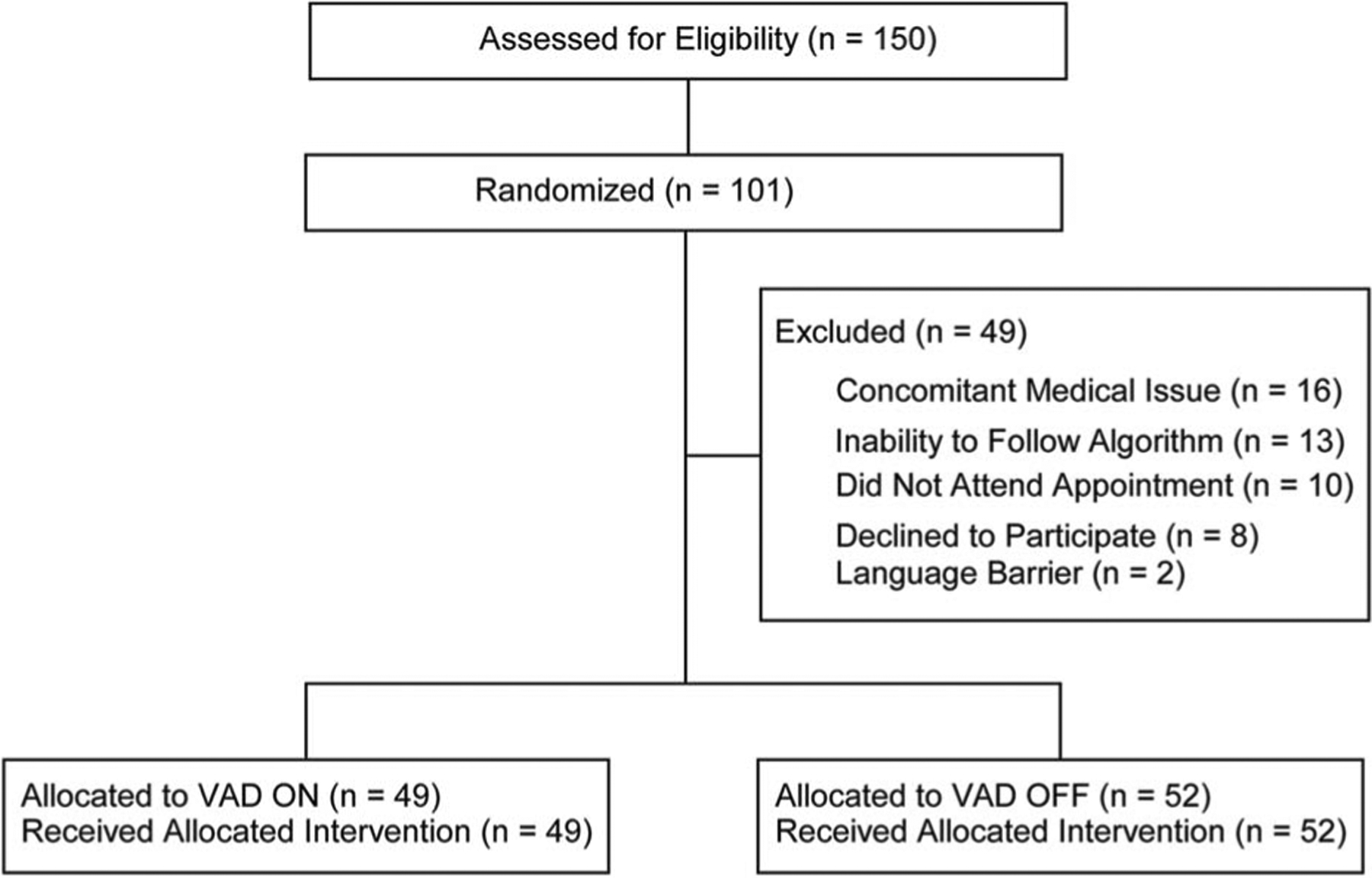
CONSORT 2010 flow diagram. Flow diagram of patient recruitment and exclusion through the study. Note that n represents the number of sites tested (n = 101, of 87 total subjects). Inclusion criteria: Adults (18+) scheduled for cutaneous cancer removal surgery. Exclusion criteria: Procedure cancellation due to a concomitant medical issue (n = 16), inability to complete the training phase (n = 13), did not attend their appointment (n = 10), declined to participate (n = 8), or were non-English speakers (n = 2). One hundred one tested sites were randomly allocated into 2 separate cohorts: VAD ON (n = 49) and VAD OFF (n = 52). All participants who were allocated were included in the final analysis. VAD, vibratory anesthetic device.
