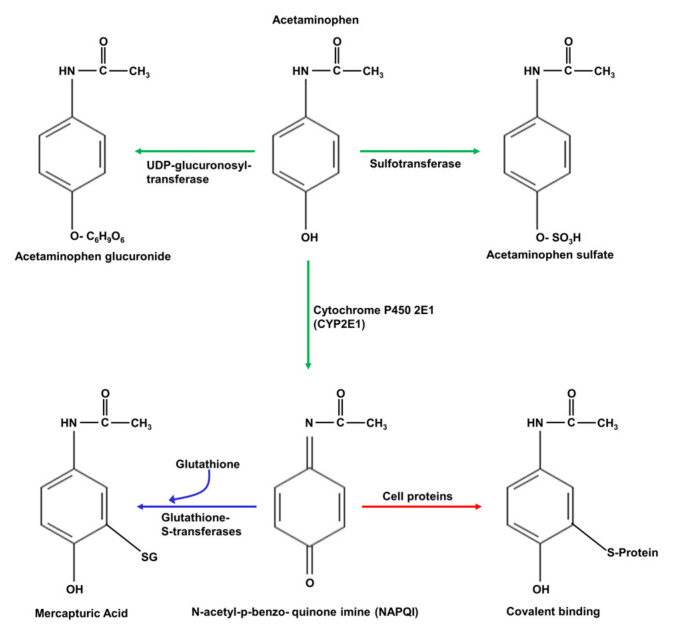
Figure 4.
The pathways by which acetaminophen is metabolised in hepatocytes. Acetaminophen is initially metabolised by UDP-glucuronosyl transferase, sulfotransferase, and cytochrome P450 2E1 (green arrows). The resulting acetaminophen glucuronide and acetaminophen sulphate are excreted from hepatocytes via the bile canaliculus. In a reaction with glutathione (GSH), the product of cytochrome P450 2E1, N-acetyl-p-benzoquinone imine (NAPQI), is converted to mercapturic acid (blue arrow), which is also excreted in bile fluid. Acetaminophen can also be N-deacylated to form p-amino-phenol sulphate (not shown). At high doses of acetaminophen, such as in acetaminophen toxicity, the glutathione available for the conversion of NAPQI to mercapturic acid is exhausted. Consequently, NAPQI accumulates and reacts with, and inactivates, enzymes and other proteins in the liver (red arrow). Moreover, the deficiency in glutathione caused by high levels of NAPQI reduces the ability of hepatocytes to remove the ROS generated in other reactions, leading to further ROS-induced damage to proteins [64,68].