Abstract
INTRODUCTION:
Pangenotypic, all-oral direct-acting antivirals, such as glecaprevir/pibrentasvir (G/P), are recommended for treatment of hepatitis C virus (HCV) infection. Concerns exist about the impact on efficacy in patients with suboptimal adherence, particularly with shorter treatment durations. These post hoc analyses evaluated adherence (based on pill count) in patients prescribed 8- or 12-week G/P, the impact of nonadherence on sustained virologic response at post-treatment week 12 (SVR12), factors associated with nonadherence, and efficacy in patients interrupting G/P treatment.
METHODS:
Data were pooled from 10 phase 3 clinical trials of treatment-naive patients with HCV genotype 1–6 without cirrhosis/with compensated cirrhosis (treatment adherence analysis) and 13 phase 3 clinical trials of all patients with HCV (interruption analysis).
RESULTS:
Among 2,149 patients included, overall mean adherence was 99.4%. Over the treatment duration, adherence decreased (weeks 0–4: 100%; weeks 5–8: 98.3%; and weeks 9–12: 97.1%) and the percentage of patients with ≥80% or ≥90% adherence declined. SVR12 rate in the intention-to-treat (ITT) population was 97.7% (modified ITT SVR12 99.3%) and remained high in nonadherent patients in the modified ITT population (<90%: 94.4%–100%; <80%: 83.3%–100%). Psychiatric disorders were associated with <80% adherence, and shorter treatment duration was associated with ≥80% adherence. Among 2,902 patients in the interruption analysis, 33 (1.1%) had a G/P treatment interruption of ≥1 day, with an SVR12 rate of 93.9% (31/33). No virologic failures occurred.
DISCUSSION:
These findings support the impact of treatment duration on adherence rates and further reinforce the concept of “treatment forgiveness” with direct-acting antivirals.
INTRODUCTION
The advent of highly curative treatments for hepatitis C virus (HCV) infection has transformed the treatment landscape for HCV. Pangenotypic, all-oral, interferon-free direct-acting antivirals (DAAs), including the 2-drug combinations of glecaprevir/pibrentasvir (G/P) and sofosbuvir/velpatasvir (SOF/VEL), are well tolerated and highly effective with relatively short treatment durations (1,2). However, despite these developments, it is estimated that most people living with HCV are unaware of their diagnosis, and only a minority of those aware are receiving treatment (3). Furthermore, historical concerns over nonadherence and its possible clinical impact endure from the pre-DAA era (4).
The underlying concerns regarding DAA nonadherence are increased risk of treatment failure and the emergence of resistance-associated substitutions that may make retreatment difficult (5-7) and hence may deter healthcare professionals from prescribing treatment for some patients (8-11). Indeed, recent evidence suggests substance use, alcohol use, and unstable housing are still perceived by treatment providers as barriers to adherence (5,12,13). Although it is acknowledged that treatment in some patient populations remains challenging, there is an emergent consensus that drug use should not be considered an contraindication for HCV treatment by clinicians or insurance payers (14).
In addition to the proven efficacy of pangenotypic, all-oral DAAs, high adherence has been observed in patients at risk of poor adherence, including those with mental health conditions and substance use (6,7,15). Nevertheless, adherence to DAAs has been demonstrated to decline steadily over the course of treatment (6,8,16,17). For this reason, shorter treatment durations that do not compromise sustained virologic response (SVR) rates may positively impact adherence, especially for patients who are less engaged in healthcare. Short HCV treatment duration, therefore, may be a key component to facilitate progress toward the World Health Organization goal to eliminate HCV as a major public health threat by 2030 (18).
G/P is approved to treat patients aged 12 years and older who are chronically infected with HCV genotypes (GT)1–6. High SVR12 rates were observed with the shortest approved treatment duration of 8 weeks for patients without cirrhosis and treatment-naive patients with compensated cirrhosis (1). Clinical trials and real-world studies of G/P have demonstrated low rates of discontinuation and high rates of adherence (1,19–21). With most HCV-infected patients eligible for 8-week G/P treatment, concerns have been raised as to whether suboptimal adherence or treatment interruption could negatively impact SVR12 rates, given its short treatment duration. For this reason, the “clinical forgiveness” (i.e., the ability of effective treatment to be unaffected by suboptimal treatment adherence) of the 8-week G/P regimen requires further investigation. In the current post hoc analyses, we assessed the impact of treatment duration on adherence in patients prescribed 8 or 12 weeks of G/P, the factors associated with nonadherence, and the impact of nonadherence on SVR12. In addition, we investigated efficacy outcomes in patients interrupting G/P treatment.
METHODS
Study design and patient population
These analyses included patients from a total of 13 phase 3 clinical trials, with data from 10 studies used for the treatment adherence analysis and data from 13 studies used for the treatment interruption analysis. For all included studies, written informed consent was obtained from each patient and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review committee.
Treatment adherence analysis.
Data were pooled from 10 phase 3 clinical trials of treatment-naive patients with HCV GT1–6, without cirrhosis or with compensated cirrhosis, prescribed 8- or 12-week G/P treatment regimens. These were SURVEYOR-2 (part 3 and part 4; NCT02243293) (22,23), ENDURANCE-1 (NCT02604017) (24), ENDURANCE-2 (active arm; NCT02640482) (23), ENDURANCE-3 (NCT02640157) (24), ENDURANCE-4 (NCT02636595) (23), ENDURANCE-5,6 (NCT02966795) (25), the aspartate aminotransferase-to-platelet ratio index (APRI) study (NCT03212521) (26), EXPEDITION-1 (NCT02642432) (27), EXPEDITION-2 (NCT02738138) (28), and EXPEDITION-8 (NCT03089944) (29).
The inclusion and exclusion criteria for each study have been previously reported (22–29). In brief, patients were aged 18 years and older, with chronic HCV GT1–6 infection with or without compensated cirrhosis. Patients with coinfection of multiple HCV genotypes were excluded in all studies, except the APRI study. Patients with a positive test for hepatitis B surface antigen were excluded. Patients in EXPEDITION-2 were coinfected with human immunodeficiency virus (HIV)-1 (28); patients with HIV-1 coinfection were also eligible for ENDURANCE-1 (24) and the APRI study (26), but not for the other studies. To be included in this analysis, patients were required to have an intended treatment duration of 8 or 12 weeks.
Only trial data from patients naive to newer DAA treatment (such as G/P and SOF/VEL) or those experienced with interferon or pegylated interferon treatment with or without ribavirin, or SOF plus ribavirin with or without pegylated interferon were included in this analysis to avoid biases related to previous treatment experience on subsequent outcomes, including rates of adherence and interruption.
Treatment interruption analysis.
Data were pooled from 13 phase 3 clinical trials for all patients with HCV who completed 8- or 12-week G/P treatment (≥52 days of treatment for 8-week G/P and ≥77 days of treatment for 12-week G/P). These were SURVEYOR-2 (part 3 and part 4; NCT02243293) (22,23), ENDURANCE-1 (NCT02604017) (24), ENDURANCE-2 (active arm; NCT02640482) (23), ENDURANCE-3 (NCT02640157) (24), ENDURANCE-4 (NCT02636595) (23), ENDURANCE-5,6 (NCT02966795) (25), the APRI study (NCT03212521) (26), EXPEDITION-1 (NCT02642432) (27), EXPEDITION-2 (NCT02738138) (28), EXPEDITION-4 (NCT02651194) (30), EXPEDITION-5 (NCT03069365) (31), EXPEDITION-8 (NCT03089944) (29), and MAGELLAN-2 (NCT02692703) (32).
The inclusion and exclusion criteria for each study have been previously reported (22–32). In addition to those stated above, patients in EXPEDITION-4 had chronic kidney disease stage 4 or 5 (30); patients in EXPEDITION-5 had chronic kidney disease stage 3b, 4, or 5 (31). Patients in MAGELLAN-2 were recipients of kidney or liver transplants (32). No specific exclusion criteria were applied to this post hoc analysis, and therefore, patients naive to HCV treatment or experienced to pegylated interferon plus ribavirin (with or without SOF) were included.
Endpoints and assessments
Treatment adherence analysis.
Adherence was calculated using available data on the percentage of pills taken relative to total number expected to be taken during each dispensation interval (weeks 0–4, weeks 5–8, and weeks 9–12), excluding any patient with missing pill count data for that interval. Electronic detection of bottle opening as a pill count method was not used in the study, and missing pill count data are reported in the results. Drug accountability was performed by the monitor throughout the treatment period. Final accountability was verified by the monitor on the completion of or discontinuation of the study drug treatment at the site. SVR12 was assessed in the intention-to-treat (ITT) population (all patients receiving ≥1 dose of G/P) and the modified ITT population, which excluded those who did not achieve SVR12 because of nonvirologic failure, including premature treatment discontinuation, reinfection, and missing SVR12 data.
Treatment interruption analysis.
Treatment interruption was defined as a temporary stop in G/P treatment for ≥1 day. Duration of interruption was calculated as the total number of days of interruption for each patient who temporarily stopped dosing and went on to complete treatment. Premature treatment discontinuation was defined as any patient who stopped treatment before the completion of the therapy and did not restart treatment. The rate of SVR12 was determined in the ITT population comprising all patients who received ≥1 dose of G/P.
Statistical analysis
Adherence and interruption data were summarized descriptively. Mean adherence rates and the proportions of patients who were ≥80% and ≥90% adherent were reported. In addition, median (range) number of treatment interruptions and duration were reported. Adherence rates were compared between treatment intervals, and the statistical significance was determined using 2-sample z tests (33).
Stepwise logistic regression analyses with significance level of 0.1 for entering effects and 0.1 for removing effects were used to identify factors associated with nonadherence during any time interval. The regression model included independent categorical covariates of treatment duration (8 or 12 weeks), cirrhosis status (no cirrhosis or compensated cirrhosis), sex (male or female), Black race (yes or no), Hispanic/Latino (yes or no), geographic region (Europe, North America, or rest of the world), stable opioid agonist therapy (yes or no), history of injection drug use (yes or no), presence of polypharmacy defined as using ≥5 concomitant medications (yes or no), current tobacco use (yes or no), current alcohol use (yes or no), history of psychiatric disorder (yes or no), HIV-1 coinfection (yes or no), and HCV GT (GT1 vs each GT2–6), and continuous covariates of age (year), body mass index (kg/m2), and baseline HCV RNA (log10 IU/mL).
RESULTS
Patient characteristics
Treatment adherence analysis population.
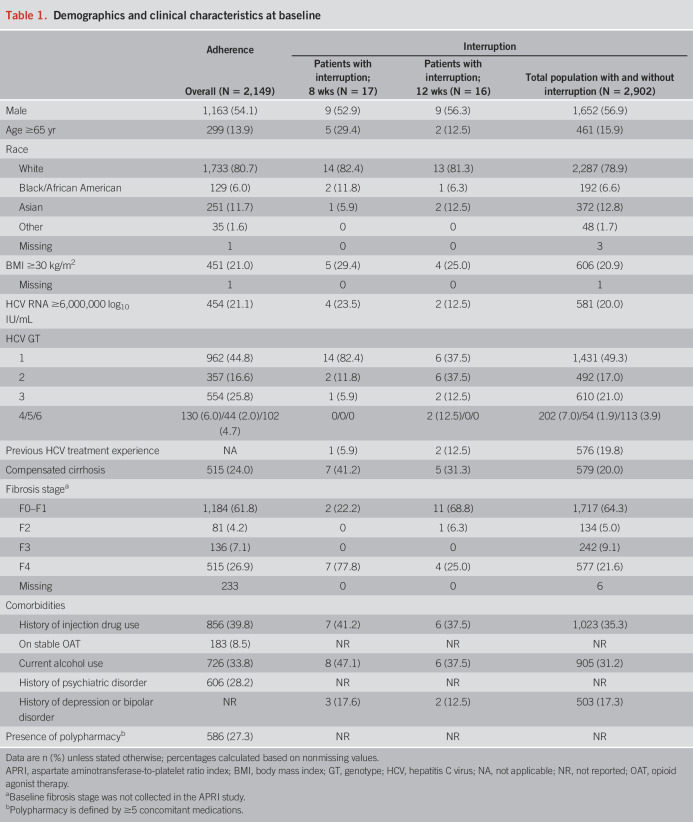
Among all 2,149 patients included in the treatment adherence analysis, median (range) age was 53.0 (19–88) years, 54.1% were men, 27.3% were receiving polypharmacy, and 39.8% had a history of injection drug use (Table 1). Among 1,634 (76.0%) patients without cirrhosis, 58.8% received 8-week treatment, and among 515 (24.0%) patients with compensated cirrhosis, 66.6% received 8-week treatment. Baseline demographics and clinical characteristics by cirrhosis status and treatment duration are provided in Supplementary Table (see Supplementary Digital Content 1, http://links.lww.com/AJG/C43); 1,304 and 845 patients were treated for 8 and 12 weeks, respectively.
Table 1.
Demographics and clinical characteristics at baseline
Treatment interruption analysis population.
Among 2,902 patients included in the treatment interruption analysis, 33 (1.1%) had a G/P treatment interruption ≥1 day and went on to complete treatment; 17 patients received 8-week treatment, and 16 patients received 12-week treatment. Baseline demographics and disease characteristics of the patients in the treatment interruption analysis population are shown in Table 1. Among the patients who had a G/P treatment interruption, 21.2% were aged 65 years and older, 54.5% were men, 36.4% had compensated cirrhosis, 39.4% had a history of injection drug use, and 90.9% were HCV treatment–naive.
Treatment adherence
Overall, 9.9% (207/2,107) of patients were nonadherent for ≥1 4-week interval. Mean treatment adherence for the overall treatment period was 99.4%. Adherence decreased as treatment length increased; mean adherence was 100% for weeks 0–4, 98.3% for weeks 5–8, and 97.1% for weeks 9–12 (Figure 1). Similar downward trends in mean adherence were seen over the 4-week intervals regardless of treatment duration or baseline cirrhosis status (see Supplementary Figure, Supplementary Digital Content 2, http://links.lww.com/AJG/C44). For the overall population, the percentage of patients with ≥80% or ≥90% adherence declined over time (Figure 2) and did not differ by treatment duration or baseline cirrhosis status (see Supplementary Figure, Supplementary Digital Content 3, http://links.lww.com/AJG/C45). Comparing weeks 5–8 with weeks 0–4, the odds ratio (OR) was 2.59 (95% confidence interval [CI] 1.29–5.20; P = 0.007) for <80% adherence and 3.74 (95% CI 2.50–5.58; P < 0.0001) for <90% adherence. Comparing weeks 9–12 with weeks 5–8, the OR was 1.86 (95% CI 1.05–3.28; P = 0.033) for <80% adherence and 1.68 (95% CI 1.24–2.29; P < 0.001) for <90% adherence.
Figure 1.
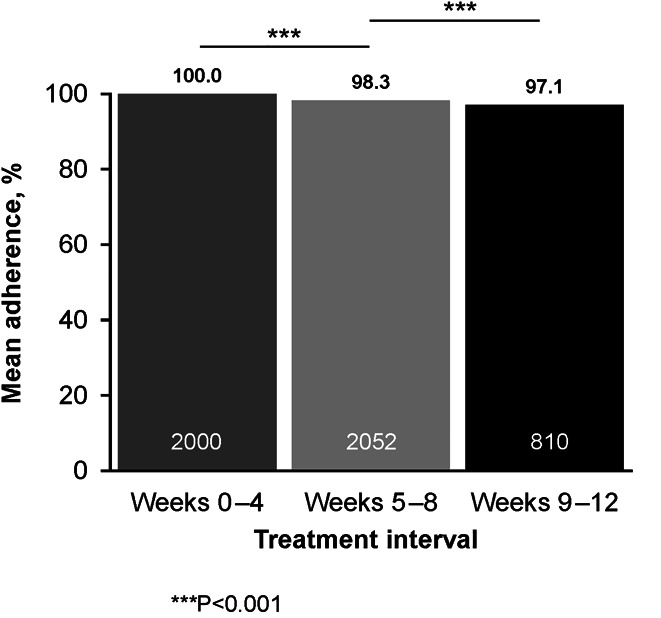
Mean adherence by treatment interval.
Figure 2.
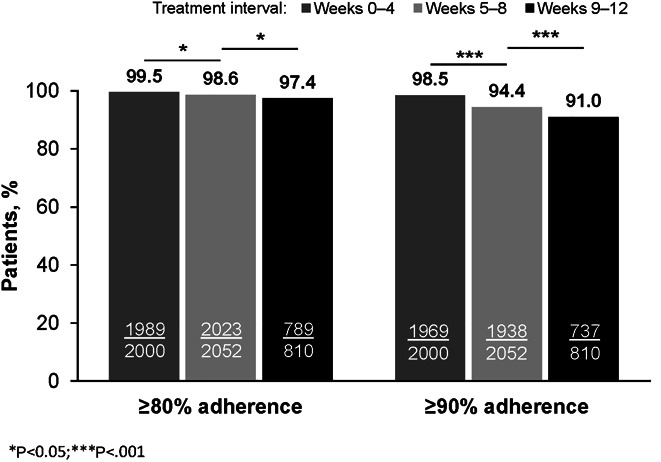
Percentage of patients with ≥80% or ≥90% adherence by treatment interval. Missing: n = 149, weeks 0–4; n = 97, weeks 5–8; and n = 1,339, weeks 9–12.
In total, there were missing pill count data for 148/2,149 (7%) patients during weeks 0–4, 97/2,149 (4.5%) patients during weeks 5–8, and 35/845 (4.1%) during weeks 9–12. No patient with missing pill count data failed treatment.
SVR12 in treatment adherence analysis population.
Overall, SVR12 in the ITT population was 97.7% (2,100/2,149; 95% CI 97.0–98.3); SVR12 in the modified ITT population was 99.3% (2,100/2,114; 95% CI 98.9–99.6). For patients with ≥90% adherence in the modified ITT population, SVR12 rates ranged from 99.3% to 99.7% for all treatment intervals, regardless of treatment duration or cirrhosis status (see Supplementary Figure, Supplementary Digital Content 4A, http://links.lww.com/AJG/C46). SVR12 rates of nonadherent patients in the modified ITT population were also universally high regardless of level of nonadherence (<90%: 94.4%–100%; <80%: 83.3%–100%). SVR12 was achieved in all but 4 patients without cirrhosis receiving G/P for 12 weeks and all but 2 patients with compensated cirrhosis receiving G/P for 12 weeks (see Supplementary Figure, Supplementary Digital Content 4, http://links.lww.com/AJG/C46). No patient who was nonadherent (<90%) to the 8-week regimen of G/P (n = 3) experienced virologic failure.
Among those treated for 8 weeks, 2 patients (0.2%) had 80%–90% adherence and 1 (0.1%) had <80% adherence during both 4-week intervals but all achieved SVR12. In the 12-week treatment group, 5 patients (0.6%) had 80%–90% adherence and 2 patients (0.2%) had <80% adherence during ≥2 4-week intervals but all achieved SVR12.
Predictors of nonadherence.
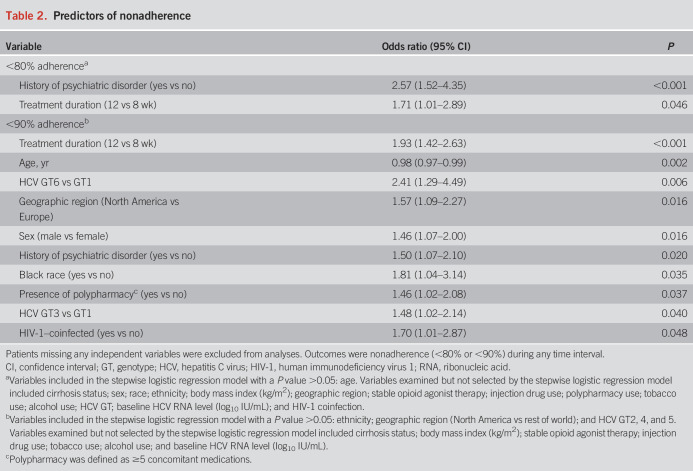
Psychiatric disorders were associated with <80% adherence (OR 2.57, 95% CI 1.52–4.35), and shorter treatment duration was associated with ≥80% adherence (OR 0.59, 95% CI 0.35–0.99) (Table 2). Ten baseline factors were statistically predictive of <90% nonadherence, with the strongest predictors being 8- vs 12-week treatment duration (OR 0.52, 95% CI 0.38–0.71), age in years (OR 0.98, 95% CI 0.97–0.99), and GT6 vs GT1 infection (OR 2.41, 95% CI 1.29–4.49).
Table 2.
Predictors of nonadherence
Treatment interruption
Overall, 1.2% (33/2,839) of patients experienced a treatment interruption of ≥1 day. Among patients with G/P treatment interruption, the median number of days for interruption was 2 (8-week G/P range 1–6; 12-week G/P range 1–62). Treatment was interrupted for ≤2 days in 23/33 (69.7%) of patients, 3–7 days in 9 patients (27.3%), and 1 patient (3.0%) with 4 interruptions totaling 62 days.
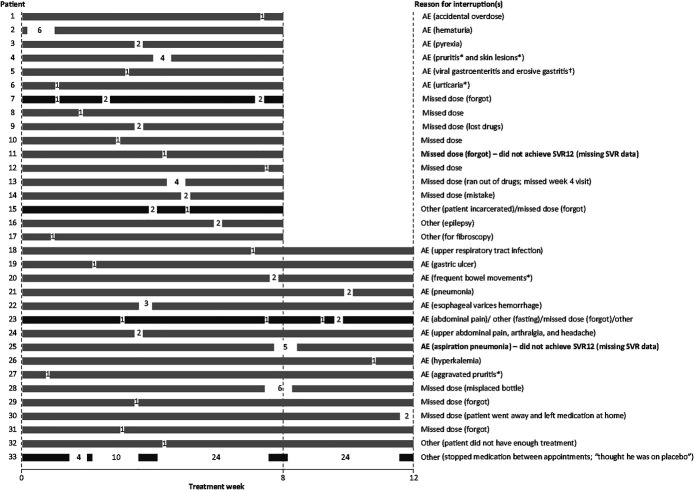
Figure 3 provides the timing, duration, and reason for each G/P treatment interruption. G/P was interrupted on multiple occasions by 4 patients. The most frequently reported reason for G/P treatment interruption was an adverse event, occurring in 16/33 patients (48.5%; 8-week G/P: 6/17 patients [35.3%]; 12-week G/P: 10/16 patients [62.5%]). Missed dose was the second most common reason for interruption occurring in 14/33 patients (42.4%; 8-week G/P: 9/17 patients [52.9%]; 12-week G/P: 5/16 patients [31.3%]). One patient with 4 treatment interruptions totaling 62 days of a planned 84-day G/P course stopped taking the medication in between study visits without telling anyone because he “thought he was on placebo”; this patient achieved SVR12 despite only taking 22 days of treatment.
Figure 3.
Details of each G/P treatment interruption for each patient. Each bar represents an individual patient; numbers represent number of days of treatment interruption; gray bars represent patients with 1 treatment interruption; black bars represent patients with multiple treatment interruptions. Bold text denotes patients with missing SVR12 data. *Reasonable possibility of relationship to study drug. †Erosive gastritis had a reasonable possibility of relationship to study drug. AE, adverse event; G/P, glecaprevir/pibrentasvir; SVR, sustained virologic response; SVR12, sustained virologic response at post-treatment week 12.
SVR12 in patients with G/P treatment interruption.
The overall SVR12 rate was 93.9% in patients interrupting 8- or 12-week G/P treatment (8-week G/P: 16/17 [95% CI 73.0–99.0]; 12-week G/P: 15/16 [95% CI 71.7–98.9]). No virologic failures occurred, and 2 patients had missing SVR12 data. One of the patients with nonvirologic failure missed a dose for 1 day and the other interrupted treatment for 5 days between days 55 and 59 of the 12-week treatment because of aspiration pneumonia, which was considered not related to DAA administration; this patient died of an unknown cause on post-treatment day 14.
SVR12 in patients with premature G/P treatment discontinuation.
A total of 38 patients prematurely discontinued treatment (8-week G/P: median treatment duration of 17 days [range 2–49 days]; 12-week G/P: median treatment duration of 50 days [range 5–83 days]), and individual data are provided in Supplementary Table (see Supplementary Digital Content 5, http://links.lww.com/AJG/C47). Excluding the patients for whom SVR12 is unknown, the overall SVR12 rate was 77% (20/26) in patients with premature treatment discontinuation (8-week G/P: 5/9; 12-week G/P: 15/17). Reasons for premature discontinuation included 16 because of adverse events, 11 noncompliant with the treatment dosing, 5 lost to follow-up, 3 because of pregnancy, and 2 on-treatment virologic failures.
DISCUSSION
Optimal adherence to HCV treatment is still perceived to be a key factor of treatment success by some treatment prescribers, but emerging data have demonstrated some degree of forgiveness for today's highly potent and effective DAA regimens, including G/P (7,8,16). In general, high adherence to DAA treatment has been observed in clinical trials (6,7) even in populations that may be considered at increased risk of nonadherence (8,17). The findings of these post hoc analyses provide additional evidence on the impact of treatment duration on adherence rates, while also providing further support for the concept of “treatment forgiveness” with modern DAA regimens. Although overall adherence rates are generally high with all-oral DAA regimens, several studies have now demonstrated that longer treatment durations lead to higher rates of nonadherence; in particular, nonadherence rates are higher the further a patient is from treatment initiation (6,7,17,34).
In accordance with our findings, the SYNERGY study showed a statistically significant decrease in adherence to ledipasvir/sofosbuvir comparing treatment in weeks 0–4 to weeks 8–12 (98.1% ± 0.9% vs 95.0% ± 1.2%; P = 0.04) (6). Similarly, the results of the SIMPLIFY study in patients with recent drug use receiving SOF/VEL reported statistically significant increased odds of nonadherence of 8% per week (95% CI 1.06–1.11) over the course of 12-week therapy (8). This increased nonadherence to SOF/VEL occurred despite incentivization of patients to return electronic blister packs. The ANCHOR study in people who inject drugs receiving 12-week SOF/VEL observed a steady decline in visit adherence from week 4 (88%) to week 8 (83%) to week 12 (70%). In the same study, medication dispensation also declined from 97% at week 4–92% at week 8, indicating that 8 of the 100 enrolled patients, at a minimum, did not receive the third month's supply of their 12-week course (17).
In our post hoc analysis of G/P clinical trials, overall adherence was high regardless of treatment duration or cirrhosis status. When examining consecutive 4-week treatment intervals, a modest, but statistically significant decline in adherence was observed the longer patients remained on treatment. Nevertheless, suboptimal adherence did not compromise SVR12 rates with 8- or 12-week G/P therapy, a finding consistent with a pooled analysis of ongoing observational studies of G/P treatment (35). The impact of poor adherence on efficacy of treatment also seems to be limited for other DAA regimens with high SVR rates reported in both SYNERGY and SIMPLIFY (6,8). In the ANCHOR study, 100 patients infected with HCV and ongoing injection drug use received a 12-week course of SOF/VEL at a harm reduction facility. The SVR12 rate (89%, 82/92) was not impacted by suboptimal adherence (P = 0.35) (17). Adherence is anticipated to be lower in real-world settings than a clinical trial setting where patients believed to be at risk of nonadherence are often excluded and in which regular study visits facilitate adherence. Nonetheless, SVR rates for G/P in real-world settings remain high (19,20).
In the current study, although high SVR rates were maintained as duration of treatment increased, there was a significantly increased risk of nonadherence associated with 12-week treatment compared with 8-week treatment. Interestingly, the current study did not observe any difference in adherence between patients with and without compensated cirrhosis. One identified patient factor associated with nonadherence from our analysis included those with a history of psychiatric disorder, although notably our analysis did not identify history of injection drug use or concomitant opioid agonist therapy as baseline factors associated with nonadherence.
These findings provide further evidence that, although adherence to G/P treatment is generally high in both 8-week and 12-week regimens, there seems to be some treatment forgiveness. The question therefore arises whether some patients are being overtreated with an 8-week course of 300/120-mg G/P. Although 8-week durations of lower G/P doses have not been examined, a dose-ranging phase 2 trial examining 12 weeks of G/P 200/120 mg or 200/40 mg (GT1/3 only) observed SVR rates >97% in GT1 noncirrhotic patients treated for 12 weeks (36). However, these lower dose combinations had lower efficacy in GT3-infected patients (SVR12 83%) (36); thus, lower doses were not studied in phase 3 in order to offer 1 fixed-dose pangenotypic combination for all patients. The lack of impact of suboptimal adherence to the 12-week duration in this analysis not surprising in light of the ability of G/P to elicit an SVR in most patients with 8 weeks of total treatment, regardless of cirrhosis status (29).
Interruption of G/P treatment was generally rare and typically did not occur for longer than 2 days with no interruptions resulting in treatment failure. Most G/P interruptions reported were related to adverse events or accidentally missed doses. Limited information is available on the impact of treatment interruption on treatment efficacy. Missed doses were analyzed as part of the SYNERGY study, where the authors reported high SVR rates (58/60 patients [96.6%]) and all patients who missed ≥2 consecutive doses achieving SVR (6). The ANCHOR study reported no statistically significant difference in SVR achieved by patients with and without treatment interruptions, with ITT SVR12 rates of 75% and 83%, respectively (17). Although concerns about relapse may exist for patients who interrupt G/P treatment, these data suggest that neither timing of missed dose nor duration of interruption are associated with virologic failure with G/P. Nonetheless, larger data sets of noncompliance are needed to confirm these findings.
There were several limitations of this study. The populations were limited by the inclusion and exclusion criteria of the clinical trials and may not represent the population of patients with HCV in the real world. Notably, only the ENDURANCE-3 trial allowed patients with active illicit drug use or positive urine drug screen at baseline. This should be considered when comparing these results with adherence studies conducted in people who use drugs. Another limitation was the small GT6 population which limits comparisons between genotypes; however, in the logistic regression, GT6 still came out as a predictor to nonadherence compared with GT1. Although 8-week and 12-week sample sizes differ and have some imbalance in patient characteristics that may impact adherence (e.g., history of injection drug use or psychiatric disorder), the adherence results for each individual duration and cirrhosis status indicate a consistent trend of increasing nonadherence over time when there is no imbalance in sample size or patient demographics (see Supplementary Figure, Supplementary Digital Content 3, http://links.lww.com/AJG/C45). Although the overall populations were large, the number of patients with interruptions was low as were the numbers of patients with extended interruptions; therefore, any conclusions regarding the outcomes of patients with treatment interruption must be taken with caution. Furthermore, as adherence was generally high, the number of patients with nonadherence (<80% or <90% adherence) was low, especially when analyzed by treatment duration and cirrhosis status. Although nonadherence did not result in lower SVR rates in this clinical trial analysis, real-world data are needed to confirm this observation.
This study provides evidence that should reassure prescribers that suboptimal treatment adherence or treatment interruption does not compromise SVR12 rates, even with the shortest duration of G/P treatment. These data may be especially useful when considering HCV treatment in patients at risk of nonadherence, supporting the use of G/P in patients that historically may have been excluded from treatment because of fears of nonadherence (7,15,21). Overall, these findings support efforts to improve access to treatment for all patients with HCV infection, including those with risk factors for poor adherence. It is anticipated that these data may help to simplify the HCV care pathway and help achieve the World Health Organization's goal of HCV elimination by 2030 (18).
CONFLICTS OF INTEREST
Guarantor of the article: Philippe J. Zamor, MD.
Specific author contributions: All authors had access to relevant data and participated in the writing, review, and approval of the manuscript. P.J.Z., A.B., D.E.D., J.F.D., A.F.L., J.J.F., D.M., R.G., C.M., D.R.N., M.C., G.P., J.K.R., R.S., E.C., E.L., and I.M.J.: conceptualization; data curation; formal analysis; investigation; methodology; writing—original draft; writing—review and editing; funding acquisition; supervision; and approval of final draft submitted. S.S.L. and Y.H.: conceptualization; data curation; formal analysis; investigation; methodology; writing—original draft; writing—review and editing; and approval of final draft submitted.
Financial support: AbbVie sponsored the study, contributed to its design, and participated in the collection, analysis, and interpretation of the data and in the writing, reviewing, and approval of the manuscript. No honoraria or payments were made for authorship. Medical writing support was provided by Jennifer Mayes, PhD, and Heather Shawcross, PhD, of Fishawack Communications; funded by AbbVie. Glecaprevir was identified by AbbVie and Enanta.
Potential competing interests: P.J.Z.: research support for AbbVie, Gilead, and Merck; speakers bureau/consultant for AbbVie and Gilead. A.B.: research grants, speaker honoraria, or advisor: AbbVie, Gilead, and Merck. D.E.D.: employee of AbbVie and owns stock/share options. J.F.D.: research support and lecturer for AbbVie, Abbott, Bristol-Myers Squibb, Gilead, Janssen, Merck Sharpe & Dohme, and Roche. A.F.L.: grant support to UCSF related to HCV: Gilead, and AbbVie. J.J.F.: research support/consultant: Abbott, AbbVie, Enanta, Gilead, GSK, Janssen, and Roche. D.M.: speaker and ad board honoraria from AbbVie. R.G.: AbbVie, Gilead, Intercept, Genefit, Genentech, Pfizer, Allergan, Bristol-Myers Squibb, Enanta, KC Pharma, and Conatus. E.C.: employee of AbbVie and may hold stock/share options. S.S.L.: employee of AbbVie and may hold stock/share options. Y.H.: employee of AbbVie and may hold stock/share options. C.M.: speaker/advisor: Merck, AbbVie, Pfizer, Intercept, and Gilead; grant support: Gilead. D.R.N.: grant/research support to University of Florida: AbbVie, Gilead, and Merck; equity holder in Target PharmaSolutions. M.C.: speaker bureau for Merck, Gilead, AbbVie, Intercept, and Roche. G.P.: advisor: AbbVie, Dicerna, Gilead, GSK, Janssen, Ιpsen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, and Spring-Bank; lecturer: AbbVie, ELPEN, Gilead, Janssen, Merck Sharp & Dohme, Novartis, Roche, and Sanofi; research support: AbbVie and Gilead; clinical trials: AbbVie, Astellas, Bayer, Celgene, Gilead, Janssen, Merck Sharp & Dohme, Noorik, Novartis, Novo Nordisk, Regulus, Roche, and Takeda. J.K.R.: consulting or speaking at educational events from AbbVie, Abivax, Gilead, Janssen, Merck, and ViiV. R.S.: educational grants from AbbVie, Bristol-Myers Squibb, Gilead, and Norgine; advisory board or speaker's honoraria for AbbVie, Bayer, and Merck. E.L.: AbbVie and Gilead. I.M.J.: grant/research support: Assembly Biosciences, Bristol-Myers Squibb, Eli Lilly, Gilead, Genfit, Enanta, and Janssen; consultant/advisor: AbbVie, Arrowhead, Atea, Assembly Biosciences, Bristol-Myers Squibb, Intercept, Janssen, Gilead, Merck, Novo Nordisk, Springbank, and Siemens.
Study Highlights.
WHAT IS KNOWN
✓ Glecaprevir/pibrentasvir (G/P) achieves high cure rates in patients with hepatitis C virus (HCV) infection.
✓ Some patients with HCV may have suboptimal adherence because of psychiatric or substance use disorders.
WHAT IS NEW HERE
✓ Adherence declined with longer treatment duration, but efficacy remained high in nonadherent patients.
✓ Psychiatric disorders were associated with decreased adherence, and shorter treatment duration was associated with increased adherence.
✓ Suboptimal treatment adherence or treatment interruption does not compromise the efficacy of G/P.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C43, http://links.lww.com/AJG/C44, http://links.lww.com/AJG/C45, http://links.lww.com/AJG/C46, and http://links.lww.com/AJG/C47
Contributor Information
Ashley Brown, Email: ashley.brown6@nhs.net.
Douglas E. Dylla, Email: douglas.dylla@abbvie.com.
John F. Dillon, Email: j.dillon@nhs.net.
Anne F. Luetkemeyer, Email: Annie.Luetkemeyer@ucsf.edu.
Jordan J. Feld, Email: Jordan.Feld@uhn.ca.
David Mutimer, Email: david.mutimer@uhb.nhs.uk.
Reem Ghalib, Email: dkz95@yahoo.com.
Eric Crown, Email: eric.crown@abbvie.com.
Sandra S. Lovell, Email: sandra.lovell@abbvie.com.
Yiran Hu, Email: yiran.hu@abbvie.com.
Christophe Moreno, Email: Christophe.Moreno@erasme.ulb.ac.be.
David R. Nelson, Email: nelsodr@ufl.edu.
Massimo Colombo, Email: mcolombo46@yahoo.it, massimo.colombo@unimi.it.
Georgios Papatheodoridis, Email: gepapath@med.uoa.gr.
Juergen K. Rockstroh, Email: Juergen.Rockstroh@ukbonn.de.
Richard Skoien, Email: Richard.Skoien@health.qld.gov.au.
Eric Lawitz, Email: lawitz@txliver.com.
Ira M. Jacobson, Email: Ira.Jacobson@nyulangone.org.
REFERENCES
- 1.AbbVie. Maviret (glecaprevir/pibrentasvir) summary of product characteristics. (https://www.ema.europa.eu/documents/product-information/maviret-epar-product-information_en.pdf). Accessed January 13, 2020.
- 2.Gilead Sciences. Epclusa (sofosbuvir/velpatasvir) summary of product characteristics. (https://www.ema.europa.eu/documents/product-information/epclusa-epar-product-information_en.pdf). Accessed January 13, 2020.
- 3.Chhatwal J, Chen Q, Bethea ED, et al. The impact of direct-acting anti-virals on the hepatitis C care cascade: Identifying progress and gaps towards hepatitis C elimination in the United States. Aliment Pharmacol Ther 2019;50:66–74. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Stepanova M, Henry L, et al. Adherence to treatment of chronic hepatitis C: From interferon containing regimens to interferon and ribavirin free regimens. Medicine (Baltimore) 2016;95:e4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang G, Patel K, Moghe A, et al. Provider perceptions of hepatitis C treatment adherence and initiation. Dig Dis Sci 2020;65:1324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen T, Townsend K, Gordon LA, et al. High adherence to all-oral directly acting antiviral HCV therapy among an inner-city patient population in a phase 2a study. Hepatol Int 2016;10:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown A, Welzel TM, Conway B, et al. Adherence to pan-genotypic glecaprevir/pibrentasvir and efficacy in HCV-infected patients: A pooled analysis of clinical trials. Liver Int 2020;40:778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham EB, Amin J, Feld JJ, et al. Adherence to sofosbuvir and velpatasvir among people with chronic HCV infection and recent injection drug use: The SIMPLIFY study. Int J Drug Policy 2018;62:14–23. [DOI] [PubMed] [Google Scholar]
- 9.Al-Khazraji A, Patel I, Saleh M, et al. Identifying barriers to the treatment of chronic hepatitis C infection. Dig Dis 2020;38:46–52. [DOI] [PubMed] [Google Scholar]
- 10.Litwin AH, Drolet M, Nwankwo C, et al. Perceived barriers related to testing, management and treatment of HCV infection among physicians prescribing opioid agonist therapy: The C-SCOPE study. J Viral Hepat 2019;26:1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solund C, Hallager S, Pedersen MS, et al. Direct acting antiviral treatment of chronic hepatitis C in Denmark: Factors associated with and barriers to treatment initiation. Scand J Gastroenterol 2018;53:849–56. [DOI] [PubMed] [Google Scholar]
- 12.Scheft H, Fontenette DC. Psychiatric barriers to readiness for treatment for hepatitis C virus (HCV) infection among injection drug users: Clinical experience of an addiction psychiatrist in the HIV-HCV coinfection clinic of a public health hospital. Clin Infect Dis 2005;40(Suppl 5):S292–6. [DOI] [PubMed] [Google Scholar]
- 13.PREP-C. Psychosocial readiness evaluation and preparation for hepatitis C treatment (PREP-C). (www.prepc.org). Accessed January 13, 2020.
- 14.Ghany MG, Marks KM, Morgan TR, et al. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020;71:686–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serper M, Evon DM, Stewart PW, et al. Medication non-adherence in a prospective, multi-center cohort treated with hepatitis C direct-acting antivirals. J Gen Intern Med 2020;35:1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham EB, Hajarizadeh B, Amin J, et al. Adherence to once-daily and twice-daily direct acting antiviral therapy for hepatitis C infection among people with recent injection drug use or current opioid agonist therapy. Clin Infect Dis 2020;71:e115–24. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal ES, Silk R, Mathur P, et al. Concurrent initiation of hepatitis C and opioid use disorder treatment in people who inject drugs. Clin Infect Dis 2020;71:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Global Hepatitis Report 2017. (http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/). Accessed January 13, 2020. [Google Scholar]
- 19.Berg T, Naumann U, Stoehr A, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C infection: Data from the German hepatitis C-registry. Aliment Pharmacol Ther 2019;49:1052–9. [DOI] [PubMed] [Google Scholar]
- 20.D'Ambrosio R, Pasulo L, Puoti M, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir in 723 patients with chronic hepatitis C. J Hepatol 2019;70:379–87. [DOI] [PubMed] [Google Scholar]
- 21.Foster GR, Dore GJ, Wang S, et al. Glecaprevir/pibrentasvir in patients with chronic HCV and recent drug use: An integrated analysis of 7 phase iii studies. Drug Alcohol Depend 2019;194:487–94. [DOI] [PubMed] [Google Scholar]
- 22.Wyles D, Poordad F, Wang S, et al. Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: A partially randomized phase 3 clinical trial. Hepatology 2018;67:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asselah T, Kowdley KV, Zadeikis N, et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol 2018;16:417–26. [DOI] [PubMed] [Google Scholar]
- 24.Zeuzem S, Foster GR, Wang S, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med 2018;378:354–69. [DOI] [PubMed] [Google Scholar]
- 25.Asselah T, Lee SS, Yao BB, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients with chronic hepatitis C virus genotype 5 or 6 infection (ENDURANCE-5,6): An open-label, multicentre, phase 3b trial. Lancet Gastroenterol Hepatol 2019;4:45–51. [DOI] [PubMed] [Google Scholar]
- 26.Fontana RJ, Lens S, McPherson S, et al. Efficacy and safety of 8 weeks of glecaprevir/pibrentasvir in treatment-naive, HCV-infected patients with apri </= 1 in a single-arm, open-label, multicenter study. Adv Ther 2019;36:3458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): A single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis 2017;17:1062–8. [DOI] [PubMed] [Google Scholar]
- 28.Rockstroh JK, Lacombe K, Viani RM, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients coinfected with hepatitis C virus and human immunodeficiency virus type 1: The EXPEDITION-2 study. Clin Infect Dis 2018;67:1010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown RS, Jr, Buti M, Rodrigues L, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naive patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J Hepatol 2020;72:441–9. [DOI] [PubMed] [Google Scholar]
- 30.Gane E, Lawitz E, Pugatch D, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med 2017;377:1448–55. [DOI] [PubMed] [Google Scholar]
- 31.Lawitz E, Flisiak R, Abunimeh M, et al. Efficacy and safety of glecaprevir/pibrentasvir in renally impaired patients with chronic HCV infection. Liver Int 2020;40(5):1032–41. [DOI] [PubMed] [Google Scholar]
- 32.Reau N, Kwo PY, Rhee S, et al. Glecaprevir/pibrentasvir treatment in liver or kidney transplant patients with Hepatitis C virus infection. Hepatology 2018;68:1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheskin D. Handbook of Parametric and Nonparametric Statistical Procedures. 3rd edn. Chapman & Hall/CRC: Boca Raton, FL, 2004. [Google Scholar]
- 34.Townsend K, Petersen T, Gordon LA, et al. Effect of HIV co-infection on adherence to a 12-week regimen of hepatitis C virus therapy with ledipasvir and sofosbuvir. AIDS 2016;30:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lampertico P, Peck-Radosavljevic M, Bondin M, et al. Addressing barriers to hepatitis C virus (HCV) elimination: Real-world outcomes in historically underserved patients with chronic HCV infection treated with glecaprevir/pibrentasvir. Poster presented at AASLD 2019. P1583.
- 36.Kwo PY, Poordad F, Asatryan A, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol 2017;67:263–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.