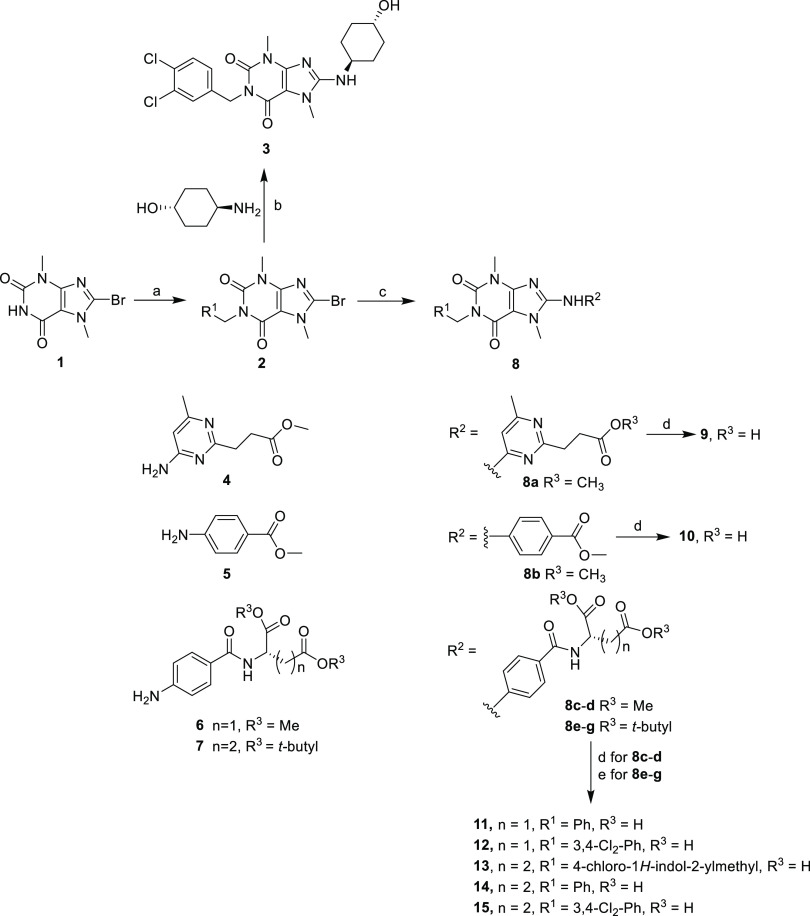
Scheme 1. Reagents and Conditions.
(a) PhCH2Br, 3.4-Cl2-PhCH2Br or 4-chloro-2-chloromethyl-1H-indole, K2CO3, DMF, 50 °C, 8 h, 72–79%; (b) trans-4-aminocyclohexanol HCl, DIPEA, 1-butanol, 120 °C, 72 h, 41%; (c) aromatic amine 4, 5, 6, or 7, Pd(OAc)2, xantphos, Cs2CO3, DMF, 120 °C, 1 h, 31–42%; (d) 2 N NaOH, DMF, 12 h, 50%; and (e) TFA, 25 °C, 0.5 h, 70–74%.