Abstract
Background
Malaria still constitutes a major public health menace, especially in tropical and subtropical countries. Close to half a million people mainly children in Africa, die every year from the disease. With the rising resistance to frontline drugs (artemisinin-based combinations), there is a need to accelerate the discovery and development of newer anti-malarial drugs. A systematic review was conducted to identify the African medicinal plants with significant antiplasmodial and/or anti-malarial activity, toxicity, as wells as assessing the variation in their activity between study designs (in vitro and in vivo).
Methods
Key health-related databases including Google Scholar, PubMed, PubMed Central, and Science Direct were searched for relevant literature on the antiplasmodial and anti-malarial activities of African medicinal plants.
Results
In total, 200 research articles were identified, a majority of which were studies conducted in Nigeria. The selected research articles constituted 722 independent experiments evaluating 502 plant species. Of the 722 studies, 81.9%, 12.4%, and 5.5% were in vitro, in vivo, and combined in vitro and in vivo, respectively. The most frequently investigated plant species were Azadirachta indica, Zanthoxylum chalybeum, Picrilima nitida, and Nauclea latifolia meanwhile Fabaceae, Euphorbiaceae, Annonaceae, Rubiaceae, Rutaceae, Meliaceae, and Lamiaceae were the most frequently investigated plant families. Overall, 248 (34.3%), 241 (33.4%), and 233 (32.3%) of the studies reported very good, good, and moderate activity, respectively. Alchornea cordifolia, Flueggea virosa, Cryptolepis sanguinolenta, Zanthoxylum chalybeum, and Maytenus senegalensis gave consistently very good activity across the different studies. In all, only 31 (4.3%) of studies involved pure compounds and these had significantly (p = 0.044) higher antiplasmodial activity relative to crude extracts. Out of the 198 plant species tested for toxicity, 52 (26.3%) demonstrated some degree of toxicity, with toxicity most frequently reported with Azadirachta indica and Vernonia amygdalina. These species were equally the most frequently inactive plants reported. The leaves were the most frequently reported toxic part of plants used. Furthermore, toxicity was observed to decrease with increasing antiplasmodial activity.
Conclusions
Although there are many indigenous plants with considerable antiplasmodial and anti-malarial activity, the progress in the development of new anti-malarial drugs from African medicinal plants is still slothful, with only one clinical trial with Cochlospermum planchonii (Bixaceae) conducted to date. There is, therefore, the need to scale up anti-malarial drug discovery in the African region.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-021-03866-0.
Keywords: Malaria, Medicinal plants, Antiplasmodial activity, Antimalarial activity
Background
Malaria still constitutes a major public health menace, especially in tropical and subtropical countries. Various species of Plasmodium, transmitted through the bite of an infected female Anopheles mosquito, cause malaria, including Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, Plasmodium vivax, and Plasmodium knowlesi. Among these species, P. falciparum is the most virulent, responsible for the highest morbidity and mortality. It is also the predominant species in sub-Saharan Africa (SSA), a region with the highest number of malaria cases and deaths in the world. According to the World Health Organization (WHO), there were 228 million cases, and 405,000 malaria attributed deaths in 2018 [1]. In SSA, children and pregnant women are the most at-risk groups [1–3].
Malaria can be treated using chemotherapy but there is widespread resistance to many of the drugs. The first case of resistance to artemisinins was reported in Cambodia in 2006 and has then spread to most of South-East Asia [4, 5]. The safety of chemoprophylaxis is also a major concern; for instance, primaquine, atovaquone, and doxycycline are contraindicated in pregnant women and children [6]. All these shortcomings necessitate the discovery and production of new drugs to treat malaria.
In the past 50 years, natural compounds including plant products, have played a major role in drug discovery and have provided value to the pharmaceutical industry [7]. For instance, therapeutics for various infectious diseases, cancer, and other debilitation diseases caused by metabolic disorders have all benefitted from many drug classes that were initially developed based on active compounds from plant sources [8]. Furthermore, quinine and artemisinin, and their synthetic derivatives which are the mainstay of anti-malarial chemotherapy, were also derived from plant sources. In malaria-endemic areas, especially in Africa, many people rely on herbal medicines as the first line of treatment [9]. The common reasons for their preference vary from the cost of standard drugs, availability and accessibility, perceived effectiveness, low side effect, and faith in traditional medicines [10].
Reviews of the antiplasmodial and anti-malarial activities of medicinal plants are needed to drive research into the discovery and production of new anti-malarial drugs. Only a few reviews of the antiplasmodial or anti-malarial activity of medicinal plants have been published in the scientific literature [11–16]. These reviews focused only on studies with high antiplasmodial or anti-malarial activity and hardly report on their toxicity. The purpose of this study was to review medicinal plants with moderate to very good antiplasmodial and anti-malarial activities, as well as assess the variation in the activities between different methods. Furthermore, the toxicity of plant species is highlighted.
Methods
The literature was reviewed in search of scientific articles reporting antiplasmodial activities (IC50, ED50, LD50, and parasite suppression rate) of medicinal plants used in Africa to treat malaria. The current study conforms to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [17].
Search strategy and selection criteria
Relevant articles were searched in health-related electronic databases including PubMed, PubMed Central, Google Scholar, and ScienceDirect using the keywords: Traditional herbs or Medicinal plants or Antiplasmodial activity or Antimalarial activity or Herbal medicine or Plasmodium.
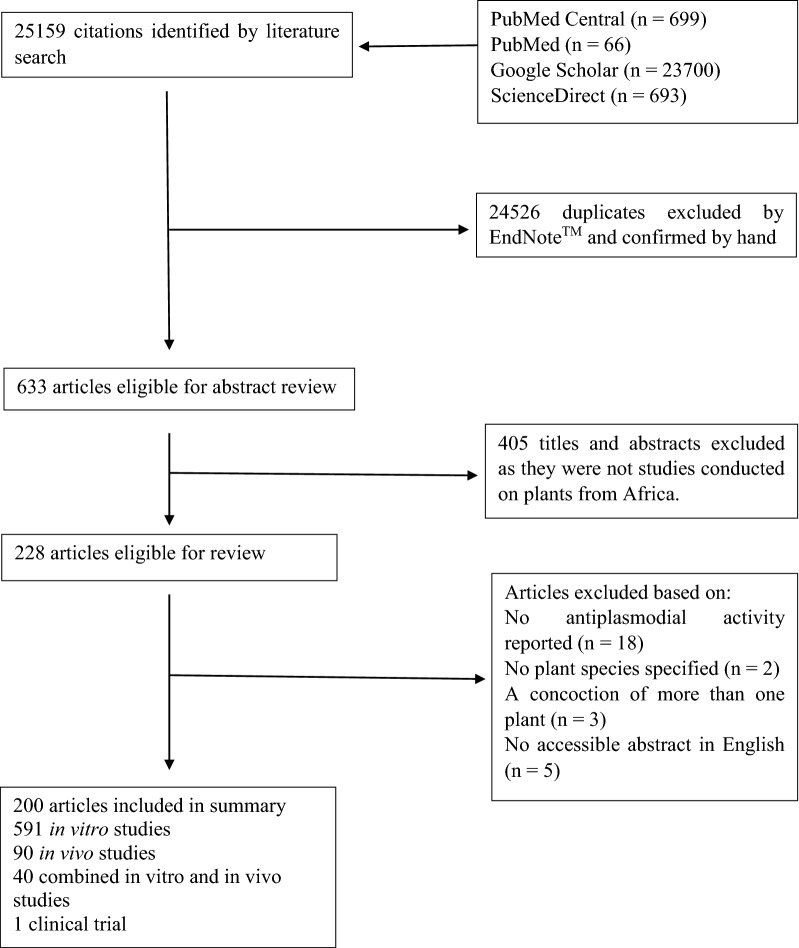
The search was limited to studies published in English or containing at least an abstract written in English until May 2020. The titles and abstracts were subsequently examined by two reviewers, independently (parallel method) to identify articles reporting the antiplasmodial activity of medicinal plants. In the case of any discrepancy in their reports, a third reviewer was brought in to resolve the issue. Relevant papers were equally manually cross-checked to identify further references. The following data were extracted from the selected articles by the reviewers: plant species, plant family, place of collection of plant, parts of the plant used, type of study (whether in vitro, in vivo, or human), the extraction solvent used, IC50 or ED50 values, parasite suppression rate, isolated compounds, interaction with known malarial drugs (whether synergistic or antagonistic), and toxicity. Articles that did not report antiplasmodial or anti-malarial activity of medicinal plants as well as review articles were excluded. The entire selection process is presented in Fig. 1.
Fig. 1.
Flowchart of the selection process for publications included in this review
In this study, antiplasmodial activity pertains to studies performed in vitro using different strains of Plasmodium falciparum, meanwhile, anti-malarial activity is reserved for in vivo studies performed using mice and various parasite models (including Plasmodium berghei, Plasmodium yoelii, and Plasmodium chabaudi) and reporting parasite suppression rate.
Categorization of antiplasmodial and anti-malarial activities
For in vitro studies, the antiplasmodial activity of an extract was considered very good if IC50 < 5 µg/ml, good 5 µg/ml ≤ IC50 < 10 µg/ml, and moderate 10 µg/ml ≤ IC50 < 20 µg/ml [18]. For in vivo studies, the anti-malarial activity of an extract is considered very good if the suppression is ≥ 50% at 100 mg/kg body weight/day, good if the suppression is ≥ 50% at 250 mg/kg body weight/day, and moderate if the suppression is ≥ 50% at 500 mg/kg body weight/day [18]. Antiplasmodial activities of 20 µg/ml and above for in vitro studies and anti-malarial ≥ 50% at > 500 mg/kg body weight/day for in vivo studies, were considered inactive.
Risk of bias in individual studies
The level of risk of bias for the study was likely to be high mainly because of differences in the studies and the methods used to determine the antiplasmodial or anti-malarial activity. The stains of Plasmodium used to assess the antiplasmodial or anti-malarial activity of the medicinal plants equally varied between studies. Furthermore, the extraction solvent, as well as the extraction yield of the plants in the different studies, was not the same, which may have accounted for the variation in the antiplasmodial and anti-malarial activities for the same plants but in the different studies.
Results
The PRISMA flowchart (Fig. 1) presents a four-phase study selection process in the present systematic review study. A total of 25,159 titles were identified in the initial search. After the title and abstract screening, 228 full-text articles were retrieved. Of these, a final 200 articles were identified for the review.
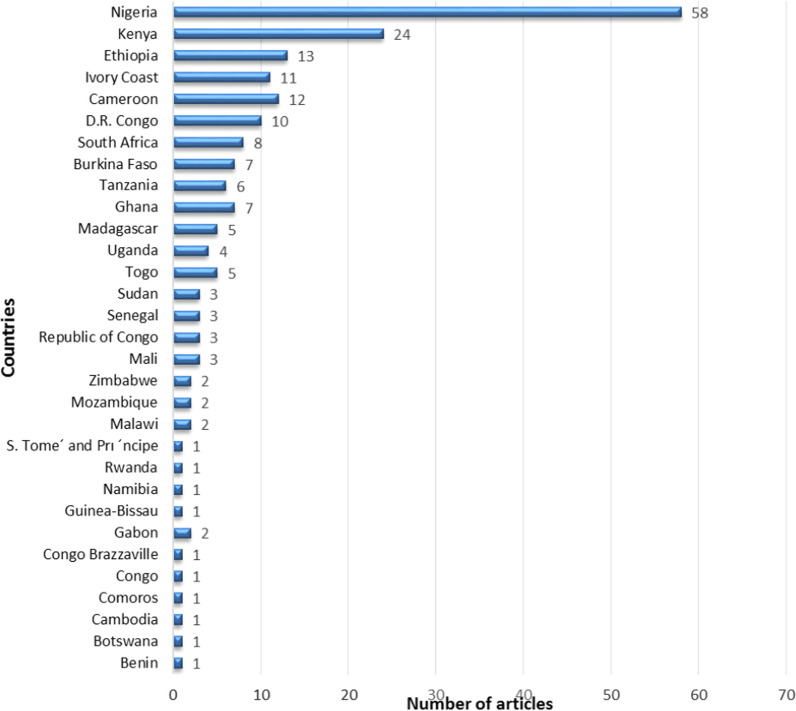
For this review, the evaluation of the individual plant species was considered as an independent study, so it is common for one article to have more than one study depending on the number of plant species evaluated. In all, there were 722 independent studies. Five hundred and ninety-on (81.9%) of the independent studies were in vitro (Table 1), 90 (12.4%) were in vivo (Table 2) and 40 (5.5%) were both in vitro and in vivo (Table 3). There was only one human study (clinical trial) conducted so far (Table 4). The selected research articles were from 31 African countries. Out of the 200 research articles reviewed, most of them were from Nigeria 58 (29.0%), Kenya 24 (12.0%), Ethiopia 13 (6.5%), Cameroon 12 (6.0%), Ivory Coast 11 (5.5%), D.R. Congo 10 (5.0%), and Burkina Faso 7 (3.5%) (Fig. 2). The studies cover the period from 1989 to 2020.
Table 1.
In vitro antiplasmodial activity of African medicinal plants
| Plant species | Plant family | Source | Country of study | Part of plant used | Extraction solvent | Antiplasmodial Activity | IC50 or ED50 or LD50 | Strain of Plasmodium Tested | Toxicity (value; assay) |
|---|---|---|---|---|---|---|---|---|---|
| Dicoma anomala subsp. Gerrardii | Compositae | [19] | South Africa | Whole plant | Methanol, Water, Hexane, Dichloromethane | Very gooda | 1.865 µM IC50 | Plasmodium falciparum 3D7, D10 | Nd |
| Abutilon grandiflorum | Malvaceae | [20] | Tanzania | Roots | Ethyl Acetate | Moderate | 10 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Acacia mellifera | Fabaceae | [21] | Kenya | Inner Barks | Methanol | Very Good | 4.48 µg/ml IC50 | Plasmodium falciparum D6 | No |
| Acacia nilotica | Fabaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 13 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [23] | Sudan | Seeds | Methanol | Very Good | 0.9–4.1 µg/ml IC50 | Plasmodium falciparum 3D7, Dd2 | No | ||
| Acacia polyacantha | Fabaceae | [20] | Tanzania | Root Barkss | Ethyl Acetate | Moderate | 13 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Acacia tortilis | Fabaceae | [24] | Kenya | Stem Barks | Methanol | Moderate | 13.4 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Very Good | 4.8 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Acacia xanthoploea | Fabaceae | [25] | South Africa | Stem Barks | Acetone | Moderate | 10.1 µg/ml IC50 | Plasmodium falciparum UP1 (CQ-R) | Nd |
| [24] | Kenya | Stem Barks | Methanol | Moderate | 17.3 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Acacia mellifera | Fabaceae | [24] | Kenya | Stem Barks | Methanol | Moderate | 12.3 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Amorpha fruticosa | Euphorbiaceae | [26] | Kenya | Leaves | Methanol | Moderate | 13.8 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Acampe pachyglossa | Orchidaceae | [20] | Kenya | Leaves | Ethyl Acetate | Moderate | 11 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Acanthospermum hispidum DC | Compositae | [27] | Burkina Faso | Stems, Leaves | Crude Alkaloid | Good | 4–10 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| [28] | Ivory Coast | Stems and Leaves | Ethanol | Moderate | 13.7 µg/ml IC50 | Plasmodium falciparum Fcb1/Colombia Strain | Nd | ||
| [29] | Republic of Congo | Leaves | Methanolic, Ethanol | Very Good | 2.8 µg/ml IC50 | Plasmodium falciparum | No | ||
| Achyranthes aspera | Amaranthaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Good | 9.9 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Acmella caulirhiza | Compositae | [30] | Kenya | Whole plant | Dichloromethane | Good | 5.201–9.939 µg/ml IC50 | Plasmodium falciparum W2, D6 | Nd |
| Acridocarpus chloropterus | Malpighiaceae | [31] | Tanzania | Roots | Dichloromethane | Good | 5.06 µg/ml IC50 | Plasmodium falciparum K1 | No |
| Achyranthes aspera | Amaranthaceae | [20] | Tanzania | Root barks | Ethyl Acetate | Very Good | 3 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Adansonia digitata | Malvaceae | [20] | Kenya | Stem barks | Ethyl Acetate | Good | 8.2 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Adenia cissampeloides | Passifloraceae | [32] | Ghana | Whole plant | Ethanol | Good | 8.521 µg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| Adhatoda latibracteata | Acanthaceae | [33] | Gabon | Stems | Dichloromethane | Very Good | 0.7–1.6 µg/ml IC50 | Plasmodium falciparum Fcbm W2 | No |
| Aerva javanica | Amaranthaceae | [34] | Sudan | Whole plant | Petroleum Ether/Chloroform | Very Good | < 5 µg/ml IC50 | Plasmodium falciparum | Nd |
| Aerva lanata | Amaranthaceae | [20] | Tanzania | Whole plant | Ethyl Acetate | Good | 8.6 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Aframomum giganteum | Zingiberaceae | [33] | Gabon | Stems | Dichloromethane | Moderate | 8.3–13.5 µg/ml IC50 | Plasmodium falciparum Fcbm W2 | No |
| Agathosma apiculata | Rutaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Good | 5.2 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Ageratum conyzoides | Compositae | [24] | Kenya | Whole plant | Methanol | Moderate | 11.5–12.1 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [30] | Kenya | Whole plant | Dichloromethane | Very Good | 2.15–3.444 µg/ml IC50 | Plasmodium falciparum W2, D6 | Nd | ||
| Ajuga remota | Lamiaceae | [35] | Kenya | Ns | Ns | Gooda | 8.2 µM IC50 | Plasmodium falciparum FCA 20/GHA | No |
| [35] | Kenya | Aerial parts | Chloroform | Good | 8.2 µg/ml IC50 | Plasmodium falciparum FCA 20/GHA | No | ||
| Alafia barteri | Apocynaceae | [36] | Nigeria | Leaves | Water | Very Good | 1.5 µg/ml IC50 | Plasmodium falciparum | Nd |
| Albizia coriaria | Fabaceae | [30] | Kenya | Stem barks | Dichloromethane | Good | 6.798–10.679 µg/ml IC50 | Plasmodium falciparum W2, D6 | Nd |
| [24] | Kenya | Stem barks | Methanol | Moderate | 15.2–16.8 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd | ||
| Albizia gummifera | Fabaceae | [24] | Kenya | Stem barks | Methanol | Good | 6.7 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [20] | Tanzania | Stem barks | Ethyl Acetate | Moderate | 15 µg/ml IC50 | Plasmodium falciparum K1 | Nd | ||
| Albizia versicolor Welw.ex Oliv | Fabaceae | [37] | South Africa | Roots | Dichloromethane | Very Good | 2.12 µg/ml IC50 | Plasmodium falciparum NF54 | Nd |
| Alchornea cordifolia | Euphorbiaceae | [38] | Ivory Coast | Leaves | Ethanol | Very Gooda | 0.2–0.5 μM IC50 | Plasmodium falciparum Fcm29 Cameroon And Nigerian Strain | No |
| [39] | Ivory Coast | Stems, leaves | Water, Ethanol, Pentane | Very Good | 2.43–4.56 µg/ml IC50 | Plasmodium falciparum Fcm29, Fcb1, Plasmodium falciparum CQ-S (Nigerian) | No | ||
| [40] | D.R.Congo | Leaves | Water | Very Good | 4.84 µg/ml IC50 | Plasmodium falciparum K1 | No | ||
| Alepidea amatymbica | Apiaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Moderate | 12.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Aloe marlothii | Xanthorrhoeaceae | [22] | South Africa | Whole plant | Dichloromethane | Very Good | 3.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Aloe ferox | Xanthorrhoeaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Good | 8 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Aloe maculata | Xanthorrhoeaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Moderate | 12.4 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Aloe pulcherrima | Xanthorrhoeaceae | [41] | Ethiopia | Roots | N-Hexane, Chloroform, Acetone Ans Methanol | Moderatea | 18.6 µg/ml IC50 | Plasmodiumfalciparum | Nd |
| Aloe secundiflora | Xanthorrhoeaceae | [24] | Kenya | Leaves | Methanol | Moderate | 15.4 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Alstonia boonei | Apocynaceae | [42] | Nigeria | Stem barks | Ethanol | Nd | nd | Plasmodium beghei NK-65 | No |
| [43] | Ivory Coast | Stem barks | Ethanol | Moderate | 12.3 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd | ||
| Alstonia congensis | Apocynaceae | [44] | D.R. Congo | Leaves, Root Barks, Stem Barks | Water, Methanol | Very Good | 2—5 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Ampelocissus africana | Vitaceae | [20] | Kenya | Whole plant | Ethyl Acetate | Good | 9.0 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Andrographis peniculata | Acanthaceae | [45] | Cambodia | Whole plant | Dichloromethane | Moderate | 12.7 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Annickia kummeriae | Annonaceae | [31] | Tanzania | Leaves | Methanol | Very Good | 0.12 µg/ml IC50 | Plasmodium falciparum K1 | No |
| Anisopappus chinensis | Compositae | [46] | D.R. Congo | Whole plant | Methanolic and dichloromethane | Good | 6.53 µg/ml IC50 | Plasmodium falciparum (3D7, W2), Plasmodium berghei berghei | No |
| Annona reticulata | Annonaceae | [47] | Cameroon | Roots | Ethanol | Very good | 1.90 µg/ml IC50 | Plasmodium falciparum W2 | No |
| Annona muricata | Annonaceae | [48] | Ivory Coast | Leaves | Pentane | Moderate | 8–18 µg/ml IC50 | Plasmodium falciparum FCM29, Plasmodium falciparum CQ-S (Nigerian) | Nd |
| [49] | Cameroon | Leaves | Hexane | Very Good | 2.03 µg/ml IC50 | Plasmodium falciparum W2 | Nd | ||
| [47] | Cameroon | Stem barks | Ethanol | Very Good | 1.45 µg/ml IC50 | Plasmodium falciparum W2 | No | ||
| Anogeissus leiocarpus | Combretaceae | [50] | Nigeria | Ns | Methanol, Water, Butanol, Ethyl Acetate | Moderate | 10.94–13.77 µg/ml IC50 | Plasmodium falciparum 3D7, K1 | Yes (SI = 121; mouse [NBMH]) |
| [51] | Ivory Coast | Leaves | Methylene Chloride | Very Good | 3.8 µg/ml IC50 | Plasmodium falciparum K1 | No | ||
| Anonidium mannii | Annonaceae | [49] | Cameroon | Twigs | Methanol | Very Good | 2.04 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Ansellia africana | Orchidaceae | [20] | Tanzania | Leaves | Ethyl Acetate | Moderate | 10 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Anthocleista grandiflora Gilg | Gentianaceae | [37] | South Africa | Stem barks | Dichloromethane | Good | 8.69 µg/ml IC50 | Plasmodium falciparum NF54 | Nd |
| Anthocleista nobilis | Gentianaceae | [52] | Burkina Faso | Leaves | Dichloromethane | Moderate | 10 µg/ml | Plasmodium falciparum | Nd |
| Anthocleista vogelii | Gentianaceae | [53] | Nigeria | Roots | Petroleum Ether | Good | 9.50 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Arenga engleri | Arecaceae | [25] | South Africa | Stem barks | Dichloromethane | Very Good | 1.7 µg/ml IC50 | Plasmodium falciparum UP1 (CQ-R) | Yes (ID50 = 35 µg/ml; Monkey kidney cells) |
| Artabotrys monteiroae | Annonaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Good | 8.7 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Artemisia afra | Asteraceae | [54] | Zimbabwe | Leaves | Petrolether/Ethylacetate | Moderate | 8.9–15.3 µg/ml IC50 | Plasmodium falciparum Pow, Dd2 | Nd |
| [22] | South Africa | Leaves | Dichloromethane | Good | 5 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| [24] | Kenya | Leaves | Methanol | Good | 3.9–9.1 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd | ||
| Artemisia annua L | Asteraceae | [24] | Kenya | Leaves | Methanol | Good | 4.7–5.5 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd |
| Artocarpus communis | Moraceae | [55] | Cameroon | Stems, Leaves | Ethanol, Water, Dichloromethane, Methanol, Hexane | Very Good | 0.67–8.20 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Asparagus virgatus | Asparagaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Good | 8 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Aspilia africana | Asteraceae | [56] | Uganda | Shoots | Ethyl Acetate | Moderate | 9.3–11.5 µg/ml IC50 | Plasmodium falciparum D10, K1 | Nd |
| Aspilia pruliseta | Compositae | [24] | Kenya | Root BARKS | Methanol | Good | 6.8–9.7 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd |
| Asystasia gangetica | Acanthaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 16 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Azadirachta indica | Meliaceae | [57] | Ivory Coast | Stems, leaves | Water | Very Good | 2.35–6.8 µg/ml IC50 | Plasmodium falciparum Fcb1 & F32 | Nd |
| [45] | Cambodia | Barks | Dichloromethane | Very Good | 4.7 µg/ml IC50 | Plasmodium falciparum W2 | Nd | ||
| [58] | Sudan | Leaves | Methanol | Very Good | 1.7–5.8 µg/ml IC50 | Plasmodium falciparum 3D7, Dd5 | Nd | ||
| [59] | Togo | Leaves | Ethanol | Very Good | 2.48–2.5 µg/ml IC50 | Plasmodium falciparum W2, D6 | Nd | ||
| Azanza garckeana | Malvaceae | [60] | Malawi | Leaves | Dichloromethane | Moderate | 11·79 µg/ml IC50 | Plasmodium falciparum, Vl/S | Nd |
| Balanites aegyptiaca | Zygophyllaceae | [24] | Kenya | Root barks | Methanol | Good | 8.9 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd |
| [21] | Kenya | Root barks | Methanol | Very good | 3.49 µg/ml IC50 | Plasmodium falciparum D6 | No | ||
| Balanites maughamii | Zygophyllaceae | [25] | South Africa | Stem barks | Dichloromethane | Very good | 1.94 µg/ml IC50 | Plasmodium falciparum UP1 (CQ-R) | Nd |
| Barringtonia racemosa | Lecythidaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Good | 5.7 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Berberis holstii | Berberidaceae | [61] | Malawi | Roots | Dichloromethane/Methanol | Very good | 0.17 µg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| [24] | Kenya | Root barks | Methanol | Very Good | < 5 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd | ||
| Bergia suffruticosa | Elatinaceae | [62] | Burkina Faso | Whole plant | Dichloromethane | Moderate | 19.53 µg/ml IC50 | Plasmodium falciparum 3D7 & W2 | Nd |
| Berula erecta | Apiaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Good | 6.6 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [24] | Kenya | Leaves | Methanol | Good | 9.9 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd | ||
| [22] | South Africa | Leaves | Methanol | Good | 5 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Bidens engleri | Compositae | [63] | Senegal | Leaves | Petroleum ether | Moderate | 9–18 µg/ml IC50 | Plasmodium falciparum FcM29, FcB1, Plasmodium vinckei petteri | Yes (IC50 = 10 µg/ml; Vero cells) |
| Bixa orellana | Bixaceae | [45] | Cambodia | Leaves | Water | Good | 9.3 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Boscia angustifolia | Capparaceae | [24] | Kenya | Stem barks | Water | Very good | 1.4–4.7 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd |
| Boscia salicifolia | Capparaceae | [26] | Kenya | Stem barks | Methanol | good | 1.1–8.8 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Boswellia dalzielii | Burseraceae | [50] | Nigeria | Ns | Methanol, Water, Butanol, Ethyl Acetate | Moderate | 14.59–15.1 µg/ml IC50 | Plasmodium falciparum 3D7, K1 | Yes (SI ≥ 101; Mouse [NBMH] |
| [62] | Burkina Faso | Leaves | Methanol | Moderate | 18.85 µg/ml IC50 | Plasmodium falciparum 3D7 & W2 | Nd | ||
| Bridelia micrantha | Phyllanthaceae | [26] | Kenya | Stem Barks | Methanol | Moderate | 14.2–19.4 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Bridelia mollis Hutch | Phyllanthaceae | [37] | South Africa | Roots | Dichloromethane | Very good | 3.06 µg/ml IC50 | Plasmodium falciparumNF54 | Nd |
| Brucea javanica | Simaroubaceae | [45] | Cambodia | Roots | Dichloromethane | Very good | 1.0 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Bruguiera gymnorhiza | Rhizophoraceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 11.7 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Burchellia bubalina | Rubiaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 18 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Caesalpinia bonducella | Fabaceae | [64] | Nigeria | Aerial Parts | Ethyl Acetate | Moderate | 16 µg/ml EC50 | Plasmodium falciparum | Yes (SI = 0.29–0.69; mouse mammary tumour [FM3A]) |
| Canthium setosum | Rubiaceae | [65] | Benin | Aerial Parts | Methylene Chloride | Very good | 2.77–4.80 µg/ml IC50 | Plasmodium falciparum 3D7 & K1 | Nd |
| Capparis tomentosa Lam | Capparaceae | [37] | South Africa | Roots | Dichloromethane | Very good | 2.19 µg/ml IC50 | Plasmodium falciparum NF54 | Nd |
| Cardiospermum halicacabum | Sapindaceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Moderate | 20 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Carica papaya | Caricaceae | [66] | Nigeria | Leaves | Ethyl Acetate | Very good | 2.96 µg/ml IC50 | Plasmodium falciparum D10, DD2 | No |
| Carissa edulis | Apocynaceae | [21] | Kenya | Root barks | Methanol | Good | 6.41 µg/ml IC50 | Plasmodium falciparum D6 | No |
| Carpolobia alba | Polygalaceae | [53] | Nigeria | Roots | Dichloromethane | Good | 7.10 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Cassia abbreviata | Fabaceae | [60] | Malawi | Roots | Dichloromethane | Very Good | 2·88 µg/ml IC50 | Plasmodium falciparum Vl/S | Nd |
| Cassia alata | Fabaceae | [67] | D.R.Congo | Leaves | Ethanol, Methanol, Petroleum Ether, Chloroform | Very Good | < 0.1—5.4 µg/ml IC50 | Plasmodium Falciparum | Nd |
| Senna occidentalis L | Fabaceae | [68] | Mozambique And Portugal | Roots | N-Hexane | Moderate | 19.3 µg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| [26] | Kenya | Root Barks | Methanol | Moderate | 18.8 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| [69] | D.R. Congo | Leaves | Petroleum Ether | Very Good | 1.5 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| [67] | D.R. Congo | Leaves | Ethanol, Methanol, Petroleum Ether, Chloroform | Very Good | < 0.1—0.25 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| Cassia siamea | Fabaceae | [70] | Togo | Leaves | Water | Good | < 7 µg/ml IC50 | Plasmodium falciparum | Nd |
| [27] | Burkina Faso | Leaves | Crude Alkaloid | Good | 4–10 µg/ml IC50 | Plasmodium falciparum W2 | Nd | ||
| Cassia tora | Fabaceae | [23] | Sudan | Aerial parts | Methanol | Good | 3.3–5.2 µg/ml IC50 | Plasmodium falciparum 3D7, Dd2 | No |
| Catha edulis | Celastraceae | [22] | South Africa | Roots | Dichloromethane | Very Good | 0.68 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Cedrelopsis grevei | Rutaceae | [71] | Madagascar | Leaves | Water | Moderate | 17.5 mg/L IC50 | Plasmodium falciparum | Nd |
| Celtis integrifolia | Cannabaceae | [52] | Burkina Faso | Leaves | Dichloromethane | Very Good | 3.7 µg/ml IC50 | Plasmodiumfalciparum | Yes (SI ≥ 0.5; HepG2 cells) |
| Centella asiatica | Apiaceae | [22] | South Africa | Leaves | Dichloromethane/Methanol | Good | 8.3 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [72] | Kenya | Root Barks | Dichloromethane | Moderate | 14.9–15.4 µg/ml IC50 | Plasmodium falciparum K1, NF54 | Nd | ||
| Cephalanthus natalensis | Rubiaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 16.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Ceratotheca sesamoides | Pedaliaceae | [63] | Senegal | Leaves | Petroleum ether | Moderate | 15–23 µg/ml IC50 | Plasmodium falciparum FcM29, FcB1, Plasmodium vinckei petteri | Yes (IC50 = 50 µg/ml; Vero cells) |
| Chrysophyllum perpulchrum | Sapotaceae | [43] | Ivory Coast | Stem Barks | Ethanol | Moderate | 12.8 µg/ml IC50 | Plasmodium falciparumFCB1 | Nd |
| Cinchona succirubra | Rubiaceae | [73] | S. Tome´ And Prı ´Ncipe | Barks | Petroleum Ether, Dichloromethane, Ethyl Acetate, Methanol | Good | < 10 µg/ml IC50 | Plasmodium falciparum3D7 And Dd2 | Nd |
| Cinnamonum camphora | Lauraceae | [57] | Ivory Coast | Cortex | Water | Moderate | 9.37–16.6 µg/ml IC50 | Plasmodium falciparumFcb1 & F32 | Nd |
| Cissampelos mucronata | Menispermaceae | [20] | Tanzania | Roots | Ethyl Acetate | Very Good | 0.38 µg/ml IC50 | Plasmodium falciparumK1 | Nd |
| [26] | Kenya | Leaves | Methanol | Very Good | 4.4 µg/ml IC50 | Plasmodium falciparumD6, W2 | Nd | ||
| Cissampelos pareira | Menispermaceae | [24] | Kenya | Root Barks | Methanol | Good | 5.2–6.5 µg/ml C50 | Plasmodium falciparumD6, W2 | Nd |
| [74] | Kenya | Root | Methanol | Good | 5.85–7.70 µg/ml IC50 | Plasmodium falciparumNF54, ENT30 | Nd | ||
| Cissus populnea | Vitaceae | [50] | Nigeria | Ns | Methanol, Water, Butanol, Ethyl Acetate | Moderate | 15.81–19.91 µg/ml IC50 | Plasmodium falciparum3D7, K1 | Yes (SI ≥ 84, Mouse [NBMH]) |
| Citropsis articulata | Rutaceae | [75] | Uganda | Root Barks | Ethyl Acetate | Nd | nd | Plasmodium falciparumFcb1 | Nd |
| Clausena anisota | Rutaceae | [24] | Kenya | Stem Barks | Methanol | Good | 8.4–9.2 µg/ml C50 | Plasmodium falciparumD6, W2 | Nd |
| [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 18 µg/ml IC50 | Plasmodium falciparumD10 | Nd | ||
| Clematis brachiata Thunb | Ranunculaceae | [37] | South Africa | Roots | Dichloromethane | Good | 5.36 µg/ml IC50 | Plasmodium falciparumNF54 | Nd |
| [21] | Kenya | Root Barks | Methanol | Very Good | 4.15 µg/ml IC50 | Plasmodium falciparumD6 | No | ||
| Clerodendrum eriophyllum | Lamiaceae | [72] | Kenya | Root Barks | Dichloromethane | Very Good | 2.7–5.3 µg/ml IC50 | Plasmodium falciparumK1, NF54 | Nd |
| [24] | Kenya | Leaves | Methanol | Very Good | < 1.8–3.9 µg/ml C50 | Plasmodium falciparumD6, W2 | Nd | ||
| Clerodendrum glabrum E. Mey | Lamiaceae | [37] | South Africa | Leaves | Dicloromethane | Good | 8.89 µg/ml IC50 | Plasmodium falciparumNF54 | Nd |
| Clerodendrum glabrum var. glabrum | Lamiaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 19 µg/ml IC50 | Plasmodium falciparumD10 | Nd |
| Clerodendrum johnstonii | Lamiaceae | [24] | Kenya | Root Barks | Methanol | Good | 8.5 µg/ml C50 | Plasmodium falciparumD6, W2 | Nd |
| Rotheca myricoides | Lamiaceae | [76] | Kenya | Root Barks | Methanol | Good | 4.0—8.4 µg/ml IC50 | Plasmodium falciparum(K39, ENT30, NF54, V1/S) | Nd |
| [26] | Kenya | Root Barks | Methanol | Good | 4.7–8.3 µg/ml IC50 | Plasmodium falciparumD6, W2 | Nd | ||
| [20] | Tanzania | Root Barks | Ethyl Acetate | Moderate | 11 µg/ml IC50 | Plasmodium falciparumK1 | Nd | ||
| [72] | Kenya | Root Barks | Dichloromethane | Moderate | 10.9–15.8 µg/ml IC50 | Plasmodium falciparumK1, NF54 | Nd | ||
| Clerodendrum rotundifolium | Lamiaceae | [24] | Kenya | Leaves | Dichloromethane | Good | < 3.9–15.7 µg/ml C50 | Plasmodium falciparumD6, W2 | Nd |
| [77] | Uganda | Leaves | Ethyl Acetate | Very Good | 0.03–0.21 µg/ml IC50 | Plasmodium falciparumNF54 & FCR3 | Nd | ||
| Clutia abyssinica | Peraceae | [24] | Kenya | Leaves | Methanol | Moderate | 7.8–11.3 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Clutia hirsuta | Peraceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Moderate | 15 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Clutia robusta | Peraceae | [24] | Kenya | Leaves | Methanol | Good | 3.4–7.5 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Cochlospermum planchonii | Bixaceae | [78] | Burkina Faso | Rhizomes | Methanol, Dichloromethane | Gooda | 2.4–11.5 μg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| [51] | Ivory Coast | Roots | Methylene Chloride | Very Good | 4.4 µg/ml IC50 | Plasmodium falciparum K1 | No | ||
| Cochlospermum tinctorium | Bixaceae | [79] | Burkina Faso | Tubecles | Ns | Very Good | 1–2 µg/ml IC50 | Plasmodium falciparum | Nd |
| [79] | Burkina Faso | Tubercles | Water | Very Good | 0.4–1.56 µg/ml IC50 | Plasmodium falciparum Fcbl And F32 | Nd | ||
| Cola caricaefolia | Malvaceae | [48] | Ivory Coast | Leaves | Pentane | Moderate | 11–16 µg/ml IC50 | Plasmodium falciparum FCM29, CQ-S (Nigerian) | No |
| Combretum collinum | Combretaceae | [52] | Burkina Faso | Leaves | Dichloromethane | Very Good | 0.2 µg/ml IC50 | Plasmodiumfalciparum | Nd |
| Combretum micranthum | Combretaceae | [57] | Ivory Coast | Stem, Leaves | Water | Very Good | 0.88–1.7 µg/ml IC50 | Plasmodium falciparum Fcb1 & F32 | Nd |
| Combretum psidioides subsp. Psilophyllum | Combretaceae | [20] | Tanzania | Root Barks | Ethyl Acetate | Good | 6.5 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Combretum zeyheri | Combretaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 15 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Commiphora africana | Burseraceae | [24] | Kenya | Stem Barks | Methanol | Good | 9.6–10.2 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Commiphora schimperi | Burseraceae | [26] | Kenya | Stem Barks | Methanol | Very Good | 3.9–5.2 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [21] | Kenya | Inner Barks | Methanol | Very Good | 4.63 µg/ml IC50 | Plasmodium falciparum D6 | No | ||
| Conyza albida | Asteraceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Very Good | 2 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Conyza podocephala | Asteraceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Good | 6.8 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Conyza scabrida | Asteraceae | [22] | South Africa | Flower | Dichloromethane/Methanol | Good | 7.8 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Copaifera religiosa | Fabaceae | [33] | Gabon | Leaves | Dichloromethane | Moderate | 8.5–13.4 µg/ml IC50 | Plasmodium falciparum FCB, 3D7 | Yes (CC50 = 4.87 µg/ml; human embryonic lung cells [MRC-5]) |
| Cordia myxa | Boraginaceae | [52] | Burkina Faso | Leaves | Dichloromethane | Good | 6.2 µg/ml IC50 | Plasmodiumfalciparum | Yes (SI = 0.5–0.9; HrpG2 cells) |
| Coula edulis | Olacaceae | [80] | Cameroon | Stem Barks | Methanol | Good | 5.79–13.8 µg/ml IC50 | Plasmodium falciparum 3D7, DD2 | No |
| Crossopteryx febrifuga | Rubiaceae | [27] | Burkina Faso | Leaves | Crude Alkaloid | Good | 4–10 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Crotalaria burkeana | Fabaceae | [22] | South Africa | Roots | Dichloromethane | Good | 9.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Croton gratissimus var. subgratissimus | Euphorbiaceae | [22] | South Africa | Leaves | Dichloromethane | Very Good | 3.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Croton lobatus | Euphorbiaceae | [65] | Benin | Roots | Methanol | Good | 2.80–6.56 µg/ml IC50 | Plasmodium falciparum 3D7 & K1 | Nd |
| Croton macrostachyus | Euphorbiaceae | [30] | Kenya | Leaves, Stems | Dichloromethane | Very Good | 2.72 µg/ml IC50 | Plasmodium falciparum W2, D6 | Nd |
| Croton menghartii | Euphorbiaceae | [22] | South Africa | Leaves | Dichloromethane/Methanol | Very Good | 1.7 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Croton pseudopulchellus | Euphorbiaceae | [25] | South Africa | Stem Barks | Chloroform | Very Good | 3.45 µg/ml IC50 | Plasmodium falciparum UP1 (CQ-R) | Nd |
| Croton zambesicus | Euphorbiaceae | [55] | Cameroon | Stem Barks | Ethanol, Water, Dichloromethane, Methanol, Hexane | Good | 0.88–9.14 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| [34] | Sudan | Fruits | Petroleum Ether/Chloroform | Very Good | < 5 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| Cryptolepis sanguinolenta | Apocynaceae | [81] | Guinea-Bissau | Leaves, Roots | Ethanol, Chcl3, Chloroform | Very Good | 1.79 µg/ml IC50 | Plasmodium falciparum K1, T996 | Nd |
| [82] | Ghana | Roots | Ethanol | Very gooda | 0.031 µg/ml IC50 | Plasmodium falciparum K1, Plasmodium berghei | Nd | ||
| [83] | D.R. Congo | Root barks | Water, ethanol, chloroform | Very good | 27–41 ng/ml IC50 | Plasmodium falciparum D6, K1, W2, Plasmodium berghei yoelii, Plasmodium berghei berghei | Nd | ||
| [84] | Ghana | Roots | Hexane, ethanol, dichloromethane | Very gooda | 0.2–0.6 μM IC50 | Plasmodium vinckei petteri, Plasmodium berghei ANKA | Nd | ||
| Cussonia spicata Thunb | Araliaceae | [22] | South Africa | Fruits | Dichloromethane/Methanol | Moderate | 14 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [37] | South Africa | Root Barks | Dichloromethane | Very Good | 3.25 µg/ml IC50 | Plasmodium falciparum NF54 | Nd | ||
| Cussonia zimmermannii | Araliaceae | [20] | Tanzania | Root Barks | Petroleum Ether | Very Good | 3.3 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Cuviera longiflora | Rubiaceae | [80] | Cameroon | Leaves | Dichloromethane/Methanol | Moderate | 13.91–20.24 µg/ml IC50 | Plasmodium falciparum 3D7, DD2 | No |
| Cyathala prostate | Amaranthaceae | [43] | Ivory Coast | Whole Plant | Ethanol | Moderate | 12.4 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd |
| Cyathula schimperiana | Amaranthaceae | [24] | Kenya | Root Barks | Methanol | Moderate | 5–17.6 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd |
| Cymbopogon validus | Poaceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Good | 5.8 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Cyperus articulatus | Cyperaceae | [24] | Kenya | Tubers | Methanol | Good | 4.8–8.7 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd |
| [74] | Kenya | Rhizomes | Methanol | Good | 4.84–8.68 µg/ml IC50 | Plasmodium falciparum NF54, ENT30 | Nd | ||
| Cyphostemma spp | Vitaceae | [86] | Namibia | Whole Plant | Methanol | Very Good | 3.276 µg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| Dacryodes edulis | Burseraceae | [80] | Cameroon | Leaves | Dichloromethane/Methanol | Good | 6.45–8.62 µg/ml IC50 | Plasmodium falciparum 3D7, DD2 | No |
| [85] | Cameroon | Root Barks | Methylene Chloride/Methanol | Very Good | 0.37 µg/ml IC50 | Plasmodiumfalciparum | No | ||
| Dichapetalum guineense | Dichapetalaceae | [65] | Benin | Leaves | Methanol | Moderate | 7.35- > 20 µg/ml IC50 | Plasmodium falciparum 3D7 & K1 | Nd |
| Dichrostachys cinerea Wight et Arn | Fabaceae | [37] | South Africa | Roots | Dichloromethane | Very Good | 2.1 µg/ml IC50 | Plasmodium falciparum NF54 | Nd |
| Dicoma tomentosa | Asteraceae | [62] | Burkina Faso | Whole Plant | Dichloromethane, Methanol | Good | 7.04–7.90 µg/ml IC50 | Plasmodium falciparum 3D7 & W2 | Nd |
| [87] | Burkina Faso | Whole plant | Dichloromethane | Very Good | 1.9–3.4 µg/ml IC50 | Plasmodium Falcipârum 3D7, W2, Plasmodium berghei | Nd | ||
| Diospyros abysinica | Ebenaceae | [75] | Uganda | Leaves | Ethyl Acetate | Nd | nd | Plasmodium falciparum Fcb2 | Nd |
| Diospyros mespiliformis | Ebeneceae | [86] | Namibia | Leaves, Roots | Methanol | Very Good | 3.179–3.523 µg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| [37] | South Africa | Roots | Dichloromethane | Very Good | 4.40 µg/ml IC50 | Plasmodium falciparum NF54 | Nd | ||
| Diospyros monbuttensis | Ebenaceae | [88] | Nigeria | Leaves | Methanol | Very Good | 3.2 nM | Plasmodium falciparum | Nd |
| Dombeya shupangae | Malvaceae | [20] | Tanzania | Root Barks | Ethyl Acetate | Good | 7.5 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Dorstenia convexa | Moraceae | [56] | Cameroon | Twigs | Ethanol, Water, Dichloromethane, Methanol, Hexane | Good | 0.28–8.95 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Dorstenia klaineana | Moraceae | [33] | Gabon | Stems | Methanol | Moderate | 16.7–17.0 µg/ml IC50 | Plasmodium falciparum Fcbm, W2 | Yes (SI = 16.2–28.89; human embryonic lung cells [MRC-5]) |
| Dracaena cambodiana | Asparagaceae | [45] | Cambodia | Stems | Dichloromethane | Good | 8.7 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Drypetes natalensis | Putranjivaceae | [31] | Tanzania | Roots | Ethanol | Very Good | 1.06 µg/ml IC50 | Plasmodium falciparum K1 | No |
| Ekebergia capensis | Meliaceae | [22] | South Africa | Fruits | Dichloromethane/Methanol | Moderate | 10 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [76] | Kenya | Stem Barks | Chloroform | Good | 3.9—13.4 µg/ml IC50 | Plasmodium falciparum K39, ENT30, NF54, V1/S | Nd | ||
| [21] | Kenya | Inner Barks | Methanol | Very Good | 3.97 µg/ml IC50 | Plasmodium falciparum D6 | No | ||
| [24] | Kenya | Stem Barks | Methanol | Moderate | 10.5 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Elaeis guineensis | Arecaceae | [32] | Ghana | Leaves | Ethanol | Very Good | 1.195 µg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| Elaeodendron buchananii | Celastraceae | [24] | Kenya | Stem Barks | Methanol | Moderate | 17.1 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Enantia chlorantha | Annonaceae | [55] | Cameroon | Stem Barks | Ethanol, Water, Dichloromethane, Methanol, Hexane | Good | 0.68–14.72 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| [40] | DR Congo | Stem Barks | Water | Good | 7.77 µg/ml IC50 | Plasmodium falciparum K1 | Yes (CC50 = 3.0 µg/ml; human embryonic lung cells [MRC-5]) | ||
| Entandrophragma angolense | Meliaceae | [89] | Cameroon | Stem Barks | Dichloromethane/Methanol | Moderate | 18.4 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Entandrophragma caudatum | Meliaceae | [25] | South Africa | Stem Barks | Dichloromethane | Very Good | 2.9 µg/ml IC50 | Plasmodium falciparum UP1 (CQ-R) | No |
| Entandrophragma palustre | Meliaceae | [46] | D.R. Congo | Stem barks | Methanol | Moderate | 15.84 µg/ml IC50 | Plasmodium falciparum 3D7, W2, Plasmodium berghei berghei | Nd |
| Erigeron floribundus | Asteraceae | [48] | Ivory Coast | Leaves | Pentane | Good | 4.3-10 µg/ml IC50 | Plasmodium falciparum FCM29, Plasmodium falciparum CQ-S (Nigerian) | Nd |
| Erioglossum edule | Sapindaceae | [45] | Cambodia | Barks | Dichloromethane | Very Good | 1.7 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Erythrina abyssinica | Fabaceae | [75] | Uganda | Barks | Ethyl Acetate | Nd | nd | Plasmodium falciparum Fcb3 | Nd |
| Erythrina lysistemon | Fabaceae | [25] | South Africa | Stem Barks | Acetone | Very Good | 4.8 µg/ml IC50 | Plasmodium falciparum UP1 (CQ-R) | Nd |
| Erythrina sacleuxii | Fabaceae | [20] | Tanzania | Root Barks | Ethyl Acetate | Very Good | 3.0 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Erythrococca anomala | Euphorbiaceae | [43] | Ivory Coast | Leaves | Ethanol | Moderate | 13.1 µg/dl IC50 | Plasmodium falciparum FCB1 | Nd |
| Euclea divinorum | Ebenaceae | [24] | Kenya | Root Barks | Methanol | Good | 6.9–12.4 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Euclea natalensis | Ebenaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Very Good | 4.6 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Eucomis autumnalis | Asparagaceae | [22] | South Africa | Bulbs | Dichloromethane/Methanol | Good | 9.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Euphorbia hirta | Euphorbiaceae | [90] | D.R. Congo | Aerial Parts | Methanol, Hexane: Ethyl Acetate | Gooda | 1.1—5.4 µg/ml IC50 | Plasmodium falciparum | No |
| [70] | D.R. Congo | Whole Plant | Petroleum Ether | Very Good | 1.2 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| Euphorbia tirucalli | Euphorbiaceae | [22] | South Africa | Leaves | Dichloromethane | Moderate | 12 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Fadogia agrestis | Rubiaceae | [27] | Burkina Faso | Leaves | Crude Alkaloid | Good | 4–10 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Fagara macrophylla | Rutaceae | [28] | Ivory Coast | Stem Barks | Ethanol | Very Good | 2.3 µg/ml IC50 | Plasmodium falciparum Fcb1/Colombia Strain | No |
| Fagaropsis angolensis | Rutaceae | [24] | Kenya | Stem Barks | Methanol | Good | 4.2–6.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Fagraea fragrans | Gentianaceae | [45] | Cambodia | Stems | Dichloromethane | Moderate | 12.8 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Ficus capraefolia | Moraceae | [52] | Burkina Faso | Leaves | Dichloromethane | Very Good | 1.8 µg/ml IC50 | Plasmodium falciparum | Yes (SI = 0.4; HepG2 cells) |
| Ficus platyhylla | Moraceae | [50] | Nigeria | Ns | Methanol, Water, Butanol, Ethyl Acetate | Moderate | 13.77–15.28 µg/ml IC50 | Plasmodium falciparum 3D7, K1 | Yes (SI ≥ 77; mouse [NBMH]) |
| Ficus sur | Moraceae | [24] | Kenya | Stem Barks | Methanol | Moderate | 8.5–15.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [76] | Kenya | Stem Barks | Chloroform, Hexane | Moderate | 9.0–19.2 µg/ml IC50 | Plasmodium falciparum K39 (CQ-S), ENT30, NF54, V1/S | Nd | ||
| Ficus thonningii | Moraceae | [29] | Republic Of Congo | Leaves | Methanol, Ethanol | Good | 9.61 µg/ml IC50 | Plasmodium falciparum | No |
| [50] | Nigeria | Ns | Methanol, Water, Butanol, Ethyl Acetate | Moderate | 14.09–25.06 µg/ml IC50 | Plasmodium falciparum 3D7, K1 | Yes (SI ≥ 103; mouse [NBMH]) | ||
| Ficus sycomorus | Moraceae | [27] | Burkina Faso | Leaves | Crude Alkaloid | Good | 4–10 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Flueggea virosa | Phyllanthaceae | [91] | Comoros | Leaves | Water/Methanol | Very Good | 2 µg/ml IC50 | Plasmodium falciparum W2 | No |
| [26] | Kenya | Stem Barks | Methanol | Very Good | 2.2–3.6 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| [22] | South Africa | Leaves, Twigs | Water | Moderate | 11.4 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Fuerstia africana | Lamiaceae | [92] | Rwanda | Leaves, Stems | Methanol | Good | 4.1–6.9 µg/ml IC50 | Plasmodium falciparum 3D7, W2 | Yes (SI = 1.9; human normal foetal lung fibroblast [WI-38) |
| [21] | Kenya | Leaves | Methanol | Very Good | 3.76 µg/ml IC50 | Plasmodium falciparum D6 | No | ||
| [24] | Kenya | Whole Plant | Methanol | Very Good | 0.9–2.4 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Funtumia elastica | Apocynaceae | [43] | Ivory Coast | Stem Barks | Ethanol | Very Good | 3.6 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd |
| [28] | Ivory Coast | Stem Barks | Ethanol | Very Good | 3.3 µg/ml IC50 | Plasmodium falciparum Fcb1/Colombia Strain | No | ||
| Funtumia latifolia | Apocynaceae | [75] | Uganda | Leaves | Ethyl Acetate | Nd | nd | Plasmodium falciparum Fcb4 | Nd |
| Garcinia kola | Clusiaceae | [67] | D.R. Congo | Seeds | Ethanol, Methanol, Petroleum Ether, Chloroform | Good | 1.02—15.75 µg/ml IC50 | Plasmodium falciparum | Nd |
| [69] | D.R. Congo | Stem Barks | Petroleum Ether | Very Good | 1.6 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| Gardenia lutea | Rubiaceae | [23] | Sudan | Leaves | Methanol | Good | 3.3–5.2 µg/ml IC50 | Plasmodium falciparum 3D7, Dd2 | No |
| Gardenia sokotensis | Rubiaceae | [62] | Burkina Faso | Leaves | Dichloromethane | Moderate | 14.01 µg/ml IC50 | Plasmodium falciparum 3D7 & W2 | Nd |
| Glinus oppositifolius | Molluginaceae | [93] | Mali | Aerial parts | Chloroform | Moderate | 15.52–18.70 µg/ml IC50 | Plasmodium falciparum W2 & 3D7 | No |
| Gloriosa superba | Colchicaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Moderate | 17 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Gnidia cuneata | Thymelaeaceae | [22] | South Africa | Stems | Dichloromethane | Moderate | 15.9 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Gnidia kraussiana var. kraussiana | Thymelaeaceae | [22] | South Africa | Leaves, Twigs | Dichloromethane/Methanol | Moderate | 10.8 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Gomphrena celosioides | Amaranthaceae | [65] | Benin | Aerial Parts | Methanol | Good | 4.26–14.97 µg/ml IC50 | Plasmodium falciparum 3D7 & K1 | Nd |
| [70] | Togo | Aerial Parts | Water | Moderate | < 15 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| [20] | Tanzania | Whole plant | Ethyl Acetate | Moderate | 15 µg/ml IC50 | Plasmodium falciparum K1 | Nd | ||
| Guiera senegalensis | Combretaceae | [57] | Ivory Coast | Stem, Leave | Water | Good | 0.79–7.03 µg/ml IC50 | Plasmodium falciparum Fcb1 & F32 | Nd |
| [94] | Mali | Roots | Chloroform | Very Gooda | < 4 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| Gutenbergia cordifolia | Asteraceae | [21] | Kenya | Leaves | Methanol | Very Good | 4.40 µg/ml IC50 | Plasmodium falciparum D6 | No |
| Gynandropsis gynandra | Cleomaceae | [20] | Tanzania | Roots | Ethyl Acetate | Moderate | 14 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| H. suaveolens | Lamiaceae | [53] | Nigeria | Leaves | Petroleum Ether | Very Good | 2.54 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Haplophyllum tuberculatum | Rutaceae | [23] | Sudan | Aerial Parts | Methanol | Very Good | 1.2–1.5 µg/ml IC50 | Plasmodium falciparum 3D7, Dd2 | No |
| Harrisonia abyssinica | Rutaceae | [58] | Sudan | Stem Barks | Methanol | Good | 4.7–10 µg/ml IC50 | Plasmodium falciparum 3D7, Dd3 | Nd |
| [72] | Kenya | Stem Barks | Dichloromethane | Good | 4.4–5.6 µg/ml IC50 | Plasmodium falciparum K1, NF54 | Nd | ||
| [26] | Kenya | Root Barks | Methanol | Good | 7.8–11.4 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| [95] | Kenya | Barks/Roots/Stem | Water | Very Good | 1.0 µg/ml IC50 | Plasmodium Knowlesi | Nd | ||
| Harrisonia perforata | Rutaceae | [45] | Cambodia | StemS | Dichloromethane | Good | 6.0 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Harungana madagascariensis | Hypericaceae | [40] | D.R.Congo | Stem Barks | Water | Good | 9.64 µg/ml IC50 | Plasmodium falciparum K1 | No |
| [20] | Tanzania | Roots | Ethyl Acetate | Very Good | 4.0 µg/ml IC50 | Plasmodium falciparum K1 | Nd | ||
| Helichrysum gymnocephalum | Asteraceae | [96] | Madagascar | Leaves | Essential Oil | In Active | 25 mg/l | Plasmodium falciparum Fcb1 | Nd |
| Helichrysum cymosum | Asteraceae | [97] | South Africa | Leaves | Water, Essential Oil | Very Gooda | 1.25 µg/ml IC50 | Plasmodium falciparum FCR-3 | Yes |
| Helichrysum nudifolium | Asteraceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Good | 6.8 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Hermannia depressa | Malvaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Good | 6.9 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Hexalobus crispiflorus | Annonaceae | [98] | Cameroon | Stem Barks | Water | Very Gooda | 2.0 µg/ml IC50 | Plasmodium falciparum W6 | Nd |
| Hippobromus pauciflorus | Sapindaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Good | 5.9 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Holarrhena floribunda | Apocynaceae | [99] | Cameroon | Stem Barkss | Water, Ethanol | Good | 1.02 − 18.53 μg/mL IC50 | Plasmodium falciparum W2,D6, FCR-3, 3D7 | Nd |
| Hoslundia opposita | Lamiaceae | [20] | Tanzania | Root Barks | Petroleum Ether | Moderate | 10 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| [26] | Kenya | Leaves | Methanol | Moderate | 15.2–25.6 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| [75] | Uganda | Leaves | Ethyl Acetate | Nd | nd | Plasmodium falciparum Fcb5 | Nd | ||
| Hunteria eburnea | Apocynaceae | [43] | Ivory Coast | Stem Barks | Ethanol | Very Good | 2.2 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd |
| Hybanthus enneaspermus | Violaceae | [65] | Benin | Aerial Parts | Methanol | Moderate | 2.57- > 20 µg/ml IC50 | Plasmodium falciparum 3D7 & K1 | Nd |
| Hymenocardia acida | Phyllanthaceae | [51] | Ivory Coast | Leaves | Methylene Chloride | Good | 6.9 µg/ml IC50 | Plasmodium falciparum K1 | Yes (SI = 6–10; rat skeletal muscle myoblast [L6]) |
| Hypericum aethiopicum | Hypericaceae | [22] | South Africa | Leaves/Flowers | Dichloromethane/Methanol | Very Good | 1.4 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Hypericum lanceolatum | Hypericaceae | [80] | Cameroon | Stem Barks | Methanol, N-Hexane, Ethyl Acetate, N-Butanol | Very Good | 3.98 µg/ml IC50 | Plasmodium falciparum W2, SHF4 | Nd |
| Hypoestes forskaolii | Acanthaceae | [24] | Kenya | Root Barks | Methanol | Good | 4.3–6.7 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Hyptis pectinata | Lamiaceae | [22] | South Africa | Leaves, Stem, Flower | Dichloromethane/Methanol | Moderate | 17.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Icacina senegalensis | Icacinaceae | [100] | Senegal | Leaves | Methanol | Good | 4.7–8 µg/ml IC50 | Plasmodium falciparum 3D7, 7G8 | No |
| Isolona hexaloba | Annonaceae | [40] | D.R. Congo | Root Barks | Water | Moderate | 15.28 µg/ml IC50 | Plasmodium falciparum K1 | No |
| Khaya grandifoliola | Meliaceae | [101] | Cameroon | Barks, Seeds | Methanol-Methylene Chloride | Gooda | 1.25—9.63 μg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Khaya senegalensis | Meliaceae | [50] | Nigeria | Ns | Methanol, Water, Butanol, Ethyl Acetate | Moderate | 15.46–28.12 µg/ml IC50 | Plasmodium falciparum 3D7, K1 | Yes (SI ≥ 69; mouse [NBMH]) |
| Kigelia africana | Bignoniaceae | [24] | Kenya | Leaves | Methanol | Moderate | 15.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [80] | Cameroon | Stem Barks | Ethyl Acetate | Moderate | 11.15 μg/mL IC50 | Plasmodium falciparum W2 | No | ||
| Kirkia wilmsii | Kirkiaceae | [22] | South Africa | Leaves | Dichloromethane/Methanol | Very Good | 3.7 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Kniphofia foliosa | Xanthorrhoeaceae | [102] | Ethiopia | Roots | Dichloromethane | Very Good | 3.8 µg/mL ED50 | Plasmodium falciparum 3D7 | No |
| Landolphia lanceolata | Apocynaceae | [103] | Congo Brazzaville | Roots | Dichloromethane | Moderate | 11 µg/ml IC50 | Plasmodium falciparum Fcm29-Cameroon | Nd |
| Lannea edulis | Anacardiaceae | [20] | Kenya | Whole Plant | Ethyl Acetate | Moderate | 17 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Lantana camara | Verbenaceae | [22] | South Africa | Leaves, Twigs | Dichloromethane/Methanol | Moderate | 11 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Leonotis mollissima | Lamiaceae | [20] | Tanzania | Leaves | Ethyl Acetate | Good | 9 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Leonotis africana | Lamiaceae | [33] | Gabon | Stems | Dichloromethane | Moderate | 15.2–27.1 µg/ml IC50 | Plasmodium falciparum Fcbm W2 | Yes (SI = 6.07–6.82; human embryonic lung cells [MRC-5]) |
| Leonotis leonurus | Lamiaceae | [22] | South Africa | Leaves, Twigs | Dichloromethane/Methanol | Good | 5.4 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Leonotis nepetifolia | Lamiaceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Moderate | 15 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Leonotis ocymifolia | Lamiaceae | [22] | South Africa | Leaves | Dichloromethane/Methanol | Good | 6.1 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Leptadenia madagascariensis | Apocynaceae | [91] | Comoros | Ns | Dichloromethane | Good | 9 µg/ml IC50 | Plasmodium falciparum W2 | No |
| Leucas calostachys | Lamiaceae | [95] | Kenya | Whole Plant | Water | Very Good | 0.79 µg/ml IC50 | Plasmodium Knowlesi | Nd |
| Leucas martinicensis | Lamiaceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Moderate | 13.3 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Lippia javanica | Verbenaceae | [24] | Kenya | Root Barks | Methanol | Good | 5.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [104] | Kenya | Roots | Dichloromethane/Ethyl Acetate | Moderate | 16.7—19.2 µg/ml IC50 | Plasmodium falciparum K39, V1/S | Nd | ||
| [22] | South Africa | Roots | Dichloromethane | Very Good | 3.8 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| [25] | South Africa | Leaves | Acetone | Very Good | 4.26 µg/ml IC50 | Plasmodium falciparum UP1 (CQ-R) | Nd | ||
| Lippia multiflora | Verbenaceae | [57] | Ivory Coast | Leaves | Water | Very Good | 1.18—2.34 µg/ml IC50 | Plasmodium falciparum Fcb1 & F32 | Nd |
| Lophira lanceolata | Ochnaceae | [52] | Burkina Faso | Leaves | Dichloromethane | Very Good | 4.7 µg/ml IC50 | Plasmodium falciparum | Nd |
| Ludwigia erecta | Onagraceae | [24] | Kenya | Whole plant | Methanol | Very Good | 0.9–1.6 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Macrostylis squarrosa | Rutaceae | [22] | South Africa | Stems | Dichloromethane/Methanol | Moderate | 16 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Maesa lanceolata | Primulaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Good | 5.9 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Markhamia lutea | Bignognaceae | [76] | Uganda | Leaves | Ethyl Acetate | Nd | Nd | Plasmodium falciparum Fcb6 | Nd |
| Maytenus heterophylla | Celastraceae | [24] | Kenya | Root barks | Methanol | Very Good | 1.8–3.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Maytenus obtusifolia | Celastraceae | [24] | Kenya | Root barks | Methanol | Good | < 1.9–5.8 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Maytenus putterlickioides | Celastraceae | [26] | Kenya | Root Barks | Methanol | Good | 4.4–10.2 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Maytenus senegalensis | Celastraceae | [58] | Sudan | Stem barks | Methanol | Nd | 3.9–10 µg/ml IC50 | Plasmodium falciparum 3D7, Dd9 | Nd |
| [26] | Kenya | Root barks | Methanol | Good | 4.7–9.8 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| [22] | South Africa | Roots | Dichloromethane | Moderate | 15.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| [20] | Tanzania | Stem barks | Ethyl Acetate | Very Good | 0.16 µg/ml IC50 | Plasmodium falciparum K1 | Nd | ||
| [31] | Tanzania | Roots | Ethanol | Very Good | 2.05 µg/ml IC50 | Plasmodium falciparum K1 | No | ||
| Maytenus undata | Celastraceae | [26] | Kenya | Leaves | Water | Very Good | 0.95–1.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Melia azedarach | Meliaceae | [46] | D.R. Congo | Leaves | Dichloromethane | Moderate | 19.14 µg/ml IC50 | Plasmodium falciparum 3D7, W2, Plasmodium berghei berghei | Nd |
| Microdesmis keayana | Pandaceae | [51] | Ivory Coast | Leaves | Methylene Chloride | Moderate | 12.2 µg/ml IC50 | Plasmodium falciparum K1 | No |
| Microglossa pyrifolia | Asteraceae | [24] | Kenya | Leaves | Methanol | Moderate | 10.4 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [77] | Uganda | Leaves | Ethyl Acetate | Very Good | 0.03–0.05 µg/ml IC50 | Plasmodium falciparum NF54 & FCR3 | Nd | ||
| [92] | Rwanda | Leaves | Dichloromethane | Very Good | 1.5–2.4 µg/ml IC50 | Plasmodium falciparum 3D7, W2 | Yes (SI = 3.2; human normal foetal lungfibroblast [WI-38]) | ||
| Mikania cordata | Compositae | [20] | Tanzania | Leaves | Ethyl Acetate | Moderate | 14 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Millettia zechiana | Fabaceae | [28] | Ivory Coast | Stem Barks | Ethanol | Moderate | 16.1 µg/ml IC50 | Plasmodium falciparum Fcb1/Colombia Strain | Nd |
| [43] | Ivory Coast | Stem Barks | Ethanol | Moderate | 14.1 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd | ||
| Momordica balsamina | Cucurbitaceae | [22] | South Africa | Stems | Dichloromethane/Methanol | Good | 5.3 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [68] | Mozambique | Aerial Parts | Ns | Very Gooda | 1 μM | Plasmodium berghei, Plasmodium falciparum | Nd | ||
| Momordica charantia | Cucurbitaceae | [88] | Nigeria | Leaves | Methanol | Very Good | 12.5 nM | Plasmodium falciparum | Nd |
| Momordica foetida | Cucurbitaceae | [77] | Uganda | Leaves | Water | Good | 0.35–6.16 µg/ml IC50 | Plasmodium falciparum NF54 & FCR3 | Nd |
| Monodora myristica | Annonaceae | [33] | Gabon | Stem | Methanol | Good | 5.5–6.1 µg/ml IC50 | Plasmodium falciparum Fcbm W2 | No |
| [49] | Cameroon | Leaves | Methanol | Good | 9.03 µg/ml IC50 | Plasmodium falciparum W2 | Nd | ||
| Morinda lucida | Rubiaceae | [74] | S. Tome´ And Prı ´Ncipe | Barks | Ethanol | Good | < 10 µg/ml IC50 | Plasmodium falciparum 3D7 and Dd2 | Nd |
| [88] | Nigeria | Leaves | Methanol | Very Good | 25 nM | Plasmodium falciparum | Nd | ||
| [53] | Nigeria | Roots | Dichloromethane | Moderate | 13.37 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Morinda morindoides | Rubiaceae | [43] | Ivory Coast | Leaves | Ethanol | Good | 9.8 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd |
| [28] | Ivory Coast | Leaves | Ethanol | Moderate | 11.6 µg/ml IC50 | Plasmodium falciparum Fcb1/Colombia Strain | Nd | ||
| Moringa oleifera | Moringaceae | [26] | Kenya | Leaves | Methanol | Moderate | 9.8 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Motandra guineensis | Apocynaceae | [43] | Ivory Coast | Leaves | Ethanol | Moderate | 16.3 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd |
| Mundulea sericea | Fabaceae | [86] | Namibia | Leaves, Shoots | Methanol | Very Good | 3.279–3.352 µg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| Mitragyna inermis | Rubiaceae | [93] | Mali | Leaves | Chloroform | Very Good | 4.36–4.82 µg/ml IC50 | Plasmodium falciparum W2 & 3D7 | No |
| Nauclea latifolia | Rubiaceae | [93] | Mali | Barks | Chloroform | Good | 5.36–6.2 µg/ml IC50 | Plasmodium falciparum W2 & 3D7 | Yes (IC50 = 50 µg/ml; BALB/C mouse) |
| [28] | Ivory Coast | Barks | Ethanol | Good | 8.9 µg/ml IC50 | Plasmodium falciparum Fcb1/Colombia Strain | No | ||
| [106] | Ivory Coast | Roots, Stem | Water | Good | 0.6–7.5 µg/ml IC50 | Plasmodium falciparum Fcb1- Colombian And Nigerian Strains | Nd | ||
| [43] | Ivory Coast | Root Barks | Ethanol | Good | 7.3 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd | ||
| Nauclea pobeguinii | Rubiaceae | [107] | D.R.Congo | Stem Barks | Ethanol | In Active | 32 µg/ml IC50 | Plasmodium falciparum, Plasmodium yeolii, Plasmodium berghei | No |
| Neoboutonia glabrescens | Euphorbiaceae | [55] | Cameroon | Leaves | Ethanol, Water, Dichloromethane, Methanol, Hexane | Good | 7.56 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Neorautanenia mitis | Fabaceae | [31] | Tanzania | Tubers | Ethanol | Very Good | 1.58 µg/ml IC50 | Plasmodium falciparum K1 | No |
| Newbouldia laevis | Bignognaceae | [108] | Togo | Leaves | Ethanol | Moderate | 12.6 µg/ml IC50 | Plasmodium falciparum | Nd |
| [109] | Nigeria | Leaves | Water | Moderate | 19.5 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| [53] | Nigeria | Roots | Dichloromethane | Good | 5.00 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Ocimum americana | Lamiaceae | [24] | Kenya | Whole Plant | Methanol | Moderate | 8.9–12.1 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Very Good | 4.2 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Ocimum basilicum | Lamiaceae | [159] | D.R. Congo | Leaves | Ethanol, Methanol, Petroleum Ether, Chloroform | Good | < 0.35–18 µg/ml IC50 | Plasmodium falciparum | Nd |
| [26] | Kenya | Leaves | Methanol | Moderate | 16.4 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Ocimum gratissimum | Lamiaceae | [30] | Kenya | Leaves, Twigs | Dichloromethane | Good | 8.616 µg/ml IC50 | Plasmodium falciparum W2, D6 | Nd |
| [40] | DR Congo | Leaves | Water | Good | 7.25 µg/ml IC50 | Plasmodium falciparum K1 | No | ||
| [26] | Kenya | Leaves | Methanol | Good | 5.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Ocimum kilimandscharicum | Lamiaceae | [30] | Kenya | Leaves, Twigs | Dichloromethane | Very Good | 0.843–1.547 µg/ml IC50 | Plasmodium falciparum W2, D6 | Nd |
| Olax gambecola | Olacaceae | [43] | Ivory Coast | Whole Plant | Ethanol | Good | 5.2 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd |
| Olea europaea | Oleaceae | [24] | Kenya | Stem Barks | Methanol | Moderate | 17.3 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [21] | Kenya | Inner Barks | Methanol | Good | 9.48 µg/ml IC50 | Plasmodium falciparum D6 | No | ||
| [22] | South Africa | Leaves | Dichloromethane/Methanol | Moderate | 12 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Opilia celtidifolia | Opiliaceae | [52] | Burkina Faso | Leaves | Dichloromethane | Very Good | 2.8 µg/ml IC50 | Plasmodium falciparum | Yes (SI = 0.4; HepG2 cells) |
| Ormocarpum trachycarpum | Fabaceae | [77] | Kenya | Stem Barks | Dichloromethane/Ethyl Acetate | Moderate | 17.5—19.6 µg/ml IC50 | Plasmodium falciparum K39, V1/S | Nd |
| Osteospermum imbricatum | Asteraceae | [22] | South Africa | Stems | Dichloromethane/Methanol | Good | 7.3 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Phyllanthus amarus | Phyllanthaceae | [53] | Nigeria | Leaves | Petroleum Ether | Very Good | 4.99 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Pachypodanthium confine | Annonaceae | [98] | Cameroon | Stem Barks | Water | Moderatea | 16.6 µg/ml IC50 | Plasmodium falciparum W3 | Nd |
| Pappea capensis Eckl.& Zeyh | Sapindaceae | [37] | South Africa | Twigs | Dichloromethane | Good | 5.47 µg/ml IC50 | Plasmodium falciparum NF54 | Nd |
| Parinari curatellifolia | Chrysobalanaceae | [22] | South Africa | Roots | Dichloromethane | Good | 5.3 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [24] | Kenya | Root Barks | Methanol | Good | 3.9–7.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| [37] | South Africa | Stem Barks | Dichloromethane | Good | 6.99 µg/ml IC50 | Plasmodium falciparum NF54 | Nd | ||
| Parinari excelsa | Chrysobalanaceae | [20] | Tanzania | Stem Barks | Ethyl Acetate | Moderate | 10 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| [75] | Uganda | Barks | Ethyl Acetate | Nd | Nd | Plasmodium falciparum Fcb7 | Nd | ||
| Parkinsonia aculeata | Fabaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Good | 9 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Pavetta corymbosa | Rubiaceae | [65] | Benin | Aerial parts | Methanol | Moderate | 5.54–20 µg/ml IC50 | Plasmodium falciparum 3D7 & K1 | Nd |
| [110] | Togo | Aerial parts | Methanol | Very Good | 2.042 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| [110] | Togo | Aerial part | Methanol | Very Good | 2.042 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| Pavetta crassipes | Rubiaceae | [27] | Burkina Faso | Leaves | Crude Alkaloid | Very Good | < 4 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| [71] | Togo | Aerial parts | Water | Good | < 7 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| Pelargonium alchemilloides | Geraniaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Moderate | 15 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Pentas lanceolata | Rubiaceae | [21] | Kenya | Root barks | Methanol | Good | 5.15 µg/ml IC50 | Plasmodium falciparum D6 | No |
| Pentas longiflora | Rubiaceae | [26] | Kenya | Root barks | Methanol | Moderate | 13.3 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Pentzia globosa | Asteraceae | [22] | South Africa | Roots | Dichloromethane | Good | 8 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Phyllanthus amarus | Phyllanthaceae | [111] | Ghana | Whole Plant | Ethanol | Moderate | 11.7 µg/ml IC50 | Plasmodium falciparum Dd2 | No |
| Phyllanthus fraternus | Phyllanthaceae | [112] | Ghana | Whole plant | Methanol | Very Good | 0.44 µg/ml IC50 | Plasmodium falciparum 3D7, W2 | No |
| Phyllanthus muellerianus | Phyllanthaceae | [28] | Ivory Coast | Leaves | Ethanol | Good | 9.4 µg/ml IC50 | Plasmodium falciparum Fcb1/Colombia Strain | No |
| [43] | Ivory Coast | Leaves | Ethanol | Moderate | 10.3 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd | ||
| Phyllanthus niruri | Phyllanthaceae | [69] | D.R.Congo | Whole Plant | Petroleum Ether | Very Good | 1.3 µg/ml IC50 | Plasmodium falciparum | Nd |
| Phyllanthus urinaria | Phyllanthaceae | [45] | Cambodia | Whole Plant | Water | Very Good | 2.4 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Physalis angulata | Solanaceae | [28] | Ivory Coast | Whole Plant | Ethanol | Good | 7.9 µg/ml IC50 | Plasmodium falciparum Fcb1/Colombia Strain | Nd |
| [43] | Ivory Coast | Whole Plant | Ethanol | Good | 7.9 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd | ||
| [44] | D.R. Congo | Leaves | Methanolic and dichloromethane | Very good | 1.27 µg/ml IC50 | Plasmodium falciparum 3D7, W2, Plasmodium berghei berghei | No | ||
| Picralima nitida | Apocynaceae | [53] | Nigeria | Roots | Ethanol | Good | 6.29 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [113] | Nigeria | Stems | Methanol | Good | 6.0–6.3 µg/ml IC50 | Plasmodium falciparum D6, W2 | No | ||
| [89] | Cameroon | Seeds | Methanol | Moderate | 10.9 µg/ml IC50 | Plasmodium falciparum W2 | Nd | ||
| [114] | Ivory Coast | Root, Stem Barks Ans Fruit Rins | Ns | Very Good | 0.188–1.581 μg/ml IC50 | Plasmodium falciparum | Nd | ||
| Piper capense | Piperaceae | [91] | Comoros | Ns | Dichloromethane | Good | 7 µg/ml IC50 | Plasmodium falciparum W2 | No |
| Piptadeniastrum africanum | Leguminosae | [40] | D.R. Congo | Stem Barks | Water | Good | 6.11 µg/ml IC50 | Plasmodium falciparum K1 | Yes (SI = 1.4–1.5; human embryonic lung cells [MRC-5]) |
| [40] | D.R.Congo | Stem Barks | Water | Good | 6.11 µg/ml IC50 | Plasmodium falciparum K1 | No | ||
| Piptostigma calophyllum | Annonaceae | [49] | Cameroon | Leaves | Methanol | Good | 6.72 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Pittosporum viridiflorum | Pittosporaceae | [24] | Kenya | Leaves | Methanol | Moderate | 17.6–18.9 µg/ml iC50 | Plasmodium falciparum D6, W2 | Nd |
| [22] | South Africa | Whole Plant | Dichloromethane | Very Good | 3 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Plumbago zeylanica | Plumbaginaceae | [22] | South Africa | Leaves | Dichloromethane | Very Good | 3 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Podocarpus latifolius | Podocarpaceae | [21] | Kenya | Root Barks | Methanol | Good | 6.43 µg/ml IC50 | Plasmodium falciparum D6 | No |
| Pollichia campestris | Caryophyllaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Good | 6.8 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Polyalthia longifolia | Annonaceae | [115] | Ghana | Stem Barks | Ethanol, N-Hexane,Dichloromethane, Ethyl Acetate, Methanol-Ethyl Acetate | Gooda | 3–6 µg/ml IC50 | Plasmodium falciparum K1 | No |
| [116] | Ghana | Stem Barks | Methanol, Chloroform, Cyclohexane, Ethyl Acetate | Gooda | 4.53–10.17 µM IC50 | Plasmodium falciparum 3D8 | Nd | ||
| Polyalthia oliveri | Annonaceae | [55] | Cameroon | Stem Barks | Ethanol, Water, Dichloromethane, Methanol, Hexane | Very Good | 4.30 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| [49] | Cameroon | Stem Barks | Methanol | Very Good | 3.43 µg/ml IC50 | Plasmodium falciparum W2 | Nd | ||
| Polyalthia suaveolens | Annonaceae | [49] | Cameroon | Twigs | Methanol | Very Good | 3.23 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Polygonatum verticillatum | Asparagaceae | [117] | Kenya | Rhizome | N-Hexane, Chloroform | Very Good | 2.33—4.62 μg/ml IC50 |
Plasmodium falciparum |
No |
| Premna chrysoclada | Lamiaceae | [26] | Kenya | Leaves | Methanol | Moderate | 11.1 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Prosopis africana | Fabaceae | [50] | Nigeria | Ns | Methanol, Water, Butanol, Ethyl Acetate | Moderate | 14.97–15.28 µg/ml IC50 | Plasmodium falciparum 3D7, K1 | Yes (SI ≥ 99; mouse heart-derived cells [NBMH]) |
| Prunus africana | Rosaceae | [24] | Kenya | Stem Barks | Methanol | Moderate | 17.3 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Pseudospondias microcarpa | Anacardiaceae | [31] | Tanzania | Roots | Ethanol | Very Good | 1.13 µg/ml IC50 | Plasmodium falciparum K1 | No |
| Psiadia punctulata | Asteraceae | [22] | South Africa | Twigs | Dichloromethane | Good | 9 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Psidium guajava | Myrtaceae | [40] | DR Congo | Leaves | Water | Good | 5.46 µg/ml IC50 | Plasmodium falciparum K1 | No |
| [20] | Tanzania | Leaves | Ethyl Acetate | Moderate | 10 µg/ml IC50 | Plasmodium falciparum K1 | Nd | ||
| Psorospermum senegalense | Hypericaceae | [63] | Burkina Faso | Leaves | Dichloromethane | Moderate | 10.03 µg/ml IC50 | Plasmodium falciparum 3D7 & W2 | No |
| Ptaeroxylon obliquum | Rutaceae | [22] | South Africa | Stems | Dichloromethane/Methanol | Good | 5.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Pterocarpus angolensis | Fabaceae | [22] | South Africa | Roots | Dichloromethane | Moderate | 10.6 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Pterocarpus erinaceus | Fabaceae | [118] | Burkina Faso | Leaves Ans Barks | Ethanol, Chloroform | Very Good | 1.93 µg/ml IC50 | Plasmodium falciparum 3D7 And Dd2 | Nd |
| Pulicaria crispa | Asteraceae | [34] | Sudan | Whole Plant | Petroleum Ether/Chloroform | Very Good | < 5 µg/ml IC50 | Plasmodium falciparum | Nd |
| Pycnanthus angolensis | Myristicaceae | [28] | Ivory Coast | Stem Barks | Ethanol | Moderate | 18.2 µg/ml IC50 | Plasmodium falciparum Fcb1/Colombia Strain | Nd |
| [74] | S. Tome´ And Prı ´Ncipe | Barks | Ethanol | Very Good | < 5 µg/ml IC50 | Plasmodium falciparum 3D7 And Dd2 | Nd | ||
| Pyrenacantha grandiflora Baill | Icacinaceae | [37] | South Africa | Roots | Dichloromethane | Good | 5.82 µg/ml IC50 | Plasmodium falciparum NF54 | Nd |
| Quassia africana | Simaroubaceae | [103] | Congo Brazzaville | Leaves | Water, Ethanol, Dichloromethane | Very Good | 0.1–2.2 µg/ml IC50 | Plasmodium falciparum Fcm29-Cameroon | Yes (IC50 = 6.7 µg/ml; KB cells) |
| [40] | D.R. Congo | Root Barks | Water | Very Good | 0.46 µg/ml IC50 | Plasmodium falciparum K1 | No | ||
| Ranunculus multifidus | Ranunculaceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Very Good | 2.3 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Rauvolfia caffra Sond | Apocynaceae | [37] | South Africa | Roots | Dichloromethane | Very Good | 2.13 µg/ml IC50 | Plasmodium falciparum NF54 | Nd |
| Rauvolfia nombasiana | Apocynaceae | [26] | Kenya | Root Barks | Methanol | Good | 9.1 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Rauvolfia vomitoria | Apocynaceae | [53] | Nigeria | Roots | Dichloromethane | Very Good | 4.78 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [28] | Ivory Coast | Barks | Ethanol | Very Good | 2.5 µg/ml IC50 | Plasmodium falciparum Fcb1/Colombia Strain | No | ||
| [43] | Ivory Coast | Root Barks | Ethanol | Very Good | 2.5 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd | ||
| Rhamnus prinoides | Rhamnaceae | [77] | Kenya | Roots | Methanol | Moderate | 15.1 µg/ml IC50 | Plasmodium falciparum K39 (CQ-S), ENT30, NF54, V1/S | Nd |
| [21] | Kenya | Root Barks | Methanol | Very Good | 3.53 µg/ml IC50 | Plasmodium falciparum D6 | No | ||
| Rhizophora mucronata | Rhizophoraceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Good | 5.6 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Ricinus communis var. communis | Euphorbiaceae | [22] | South Africa | Stems | Water | Good | 8.0 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Rubia cordifolia | Rubiaceae | [95] | Kenya | Leaves/Seeds/Stems | Methanol | Very Good | 1.20 µg/ml IC50 | Plasmodium Knowlesi | Nd |
| [24] | Kenya | Whole Plant | Methanol | Very Good | < 5 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Rumex abyssinicus | Polygonaceae | [92] | Rwanda | Roots | Water | Very Good | 3.1–4.3 µg/ml IC50 | Plasmodium falciparum 3D7, W2 | Yes (SI = 3.1; human normal foetal lung fibroblast [WI-38]) |
| Rumex crispus | Polygonaceae | [22] | South Africa | Roots | Dichloromethane | Moderate | 14 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Salacia madagascariensis | Celastraceae | [20] | Tanzania | Roots | Petroleum Ether | Very Good | 0.8 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Salvia africana-lutea | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Moderate | 15.863 µg/ml IC50 | Plasmodium falciparum FCR-3 | Nd |
| Salvia albicaulis | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Moderate | 15.833 µg/ml IC50 | Plasmodium falciparum FCR-3 | Nd |
| Salvia aurita | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Good | 8.923 µg/ml IC50 | Plasmodium falciparum FCR-3 | Nd |
| Salvia chamelaeagnea | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Good | 8.713 µg/ml IC50 | Plasmodium falciparum FCR-3 | Nd |
| Salvia dolomitica | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Good | 7.623 µg/ml IC50 | Plasmodium falciparum FCR-3 | Nd |
| Salvia garipensis | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Moderate | 13.953 µg/ml IC50 | Plasmodium falciparum FCR-3 | Nd |
| Salvia muirii | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Moderate | 11.873 µg/ml IC50 | Plasmodium falciparum FCR-3 | Nd |
| Salvia radula | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Very Good | 3.913 µg/ml IC50 | Plasmodium falciparum FCR-3 | Yes (IC50 = 20.12 µg/ml; Kidney cells) |
| Salvia repens | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Good | 8.253 µg/ml IC50 | Plasmodium falciparum FCR-3 | Nd |
| [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Moderate | 10.8 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Salvia runcinata | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Moderate | 16.613 µg/ml IC50 | Plasmodium falciparum FCR-3 | Nd |
| Salvia schlechteri | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Moderate | 17.513 µg/ml IC50 | Plasmodium falciparum FCR-3 | Nd |
| Salvia stenophylla | Lamiaceae | [120] | South Africa | Aerial Parts | Methanol/Chloroform | Good | 6.53 µg/ml IC50 | Plasmodium falciparum FCR-3 | Yes (IC50 = 12.12 µg/ml; Kidney cells) |
| Sonchus schweinfurthi | Compositae | [95] | Kenya | Barks/Roots | Methanol | Very Good | 2.10 µg/ml IC50 | Plasmodium Knowlesi | Nd |
| Scaevola plumieri | Goodeniaceae | [22] | South Africa | Twigs | Dichloromethane | Moderate | 11 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Schefflera umbellifera | Araliaceae | [22] | South Africa | Leaves | Dichloromethane/Methanol | Very Good | 3.7 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Schizozygia coffaeoides | Apocynaceae | [26] | Kenya | Leaves | Methanol | Moderate | 10.5 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Schkuhria pinnata | Compositae | [24] | Kenya | Whole Plant | Methanol | Good | 1.3–6.8 µg/ml IC50 | Plasmodium falciparumD6, W2 | Nd |
| Schrankia leptocarpa | Fabaceae | [65] | Benin | Aerial Parts | Methanol | Moderate | 3.38- > 20 µg/ml IC50 | Plasmodium falciparum 3D7 & K1 | Nd |
| Sclerocarya birrea | Anacardiaceae | [24] | Kenya | Stem Barks | Methanol | Moderate | 5.9–24.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Secamone afzelii | Apocynaceae | [65] | Benin | Aerial Parts | Methanol | Moderate | 6.48- > 20 µg/ml IC50 | Plasmodium falciparum 3D7 & K1 | Nd |
| Securidaca longipedunculata | Polygalaceae | [121] | Mali | Leaves | Dichloromethane | Good | 6.9 µg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| Securinega virosa | Phyllanthaceae | [52] | Burkina Faso | Leaves | Dichloromethane | Good | 7.1 µg/ml IC50 | Plasmodium falciparum | Nd |
| Senecio oxyriifolius | Asteraceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Moderate | 13 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Senecio stuhlmannii | Asteraceae | [56] | Uganda | Shoots | Ethyl Acetate | Moderate | 14.0–15.2 µg/ml IC50 | Plasmodium falciparum D10, K1 | Nd |
| Senna didymobotrya | Fabaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Good | 9.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Senna petersiana | Fabaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 13 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [59] | Malawi | Leaves | Methanol | Very Good | 2·67 µg/ml IC50 | Plasmodium falciparum Vl/S | Nd | ||
| Sericocomopsis hildebrandtii | Amaranthaceae | [21] | Kenya | Root Barks | Methanol | Very Good | 3.78 µg/ml IC50 | Plasmodium falciparum D6 | No |
| Setaria megaphylla | Poaceae | [22] | South Africa | Whole plant | Dichloromethane/Methanol | Very Good | 4.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Sida acuta | Malvaceae | [118] | Burkina Faso | Whole Plant | Ethanol, Chloroform, Water | Very Good | 0.87–0.92 µg/ml IC50 | Plasmodium falciparum 3D7 And Dd2 | Nd |
| [38] | Ivory Coast | Ns | Ethanol | Good | 3.9–5.4 µg/ml IC50 | Plasmodium falciparum | No | ||
| Solanum panduriforme | Solanaceae | [25] | South Africa | Leaves | Acetone | Very Good | 3.62 µg/ml IC50 | Plasmodium falciparum UP1 (CQ-R) | Nd |
| Solanecio mannii | Asteraceae | [92] | Rwanda | Leaves | Dichloromethane | Moderate | 12.7–18.2 µg/ml IC50 | Plasmodium falciparum 3D7, W2 | No |
| Spilanthes mauritiana | Asteraceae | [22] | South Africa | Stems | Dichloromethane/Methanol | Good | 5.3 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Staudtia gabonensis | Myristicaceae | [33] | Gabon | Stems | Methanol | Very Good | 0.8 µg/ml IC50 | Plasmodium falciparum Fcbm W2 | No |
| Stephania abyssinica | Menispermaceae | [24] | Kenya | Root Barks | Methanol | Good | 4.7–6.1 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Stephania rotunda | Menispermaceae | [45] | Cambodia | Tubers | Dichloromethane | Very Good | 1.0 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Struchium sparganophorum | Asteraceae | [73] | S. Tome´ And Prı ´Ncipe | Leaves | Petroleum Ether | Good | < 10 µg/ml IC50 | Plasmodium falciparum 3D7 And Dd2 | Nd |
| Strychnopsis thouarsii | Menispermaceae | [122] | Madagascar | Stem Barks | Methanol | Very Gooda | 3.1—4.2 µM | Plasmodium falciparum NF54, Plasmodium yoelli 265 BY | No |
| Strychnos henningsii | Loganiaceae | [72] | Kenya | Twigs | Methanol | Moderate | 14.6–17.9 µg/ml IC50 | Plasmodium falciparum K1, NF54 | Nd |
| Strychnos pungens | Loganiaceae | [22] | South Africa | Leaves | Dichloromethane | Moderate | 12.6 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Strychnos spinosa | Loganiaceae | [123] | Senegal | Leaves, Stem | Methanol, Water | Moderate | 15 µg/ml IC50 | Plasmodiumfalciparum | Nd |
| Strychnos icaja | Loganiaceae | [46] | D.R. congo | Root barks | Methanolic and dichloromethane | Very good | 0.69 µg/ml IC50 | Plasmodium falciparum 3D7, W2, Plasmodium berghei berghei | Nd |
| Suregada zanzibariensis | Euphorbiaceae | [26] | Kenya | Leaves | Methanol | Good | 5.8–6.7 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [124] | Kenya | Leaves | Methanol | Very Good | 1.82–4.66 µg/ml IC50 | Plasmodium falciparum D6&W2 | Nd | ||
| [124] | Kenya | Leaves | Methanol | Very Good | 1.82–4.66 µg/ml IC50 | Plasmodium falciparum D6, W2 | No | ||
| Syzygium cordatum subsp. cordatum | Myrtaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 14.7 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [37] | South Africa | Leaves | Dichloromethane | Good | 6.15 µg/ml IC50 | Plasmodium falciparum NF54 | Nd | ||
| Tabernaemontana elegans | Apocynaceae | [37] | South Africa | Roots | Dichloromethane | Very Good | 0.33 µg/ml IC50 | Plasmodium falciparum NF54 | Nd |
| Tabernaemontana pachysiphon | Apocynaceae | [26] | Kenya | Flower | Methanol | Very Good | 4.4–4.8 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Tagetes minuta | Asteraceae | [75] | Uganda | Leaves | Ethyl Acetate | Nd | Nd | Plasmodium falciparum Fcb8 | Nd |
| Tamarindus indica | Fabaceae | [23] | Sudan | Stem Barks | Methanol | Moderate | 10 µg/ml IC50 | Plasmodium falciparum 3D7, Dd2 | No |
| [110] | Togo | Fruits | Water | Very Good | 4.786 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| Tapinanthus dodoneifolius | Loranthaceae | [52] | Burkina Faso | Leaves | Methanol | Good | 5.2 µg/ml IC50 | Plasmodium falciparum | Nd |
| Tarchonanthus camphoratus | Asteraceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Good | 6 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Teclea nobilis | Rutaceae | [24] | Kenya | Stem Barks | Methanol | Moderate | 3.9–20.4 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [75] | Uganda | Barks | Ethyl Acetate | Nd | Nd | Plasmodium falciparum Fcb9 | Nd | ||
| Tecoma capensis | Bignoniaceae | [22] | South Africa | Twigs | Dichloromethane/Methanol | Moderate | 10.2 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Tectona grandis | Lamiaceae | [112] | Ghana | Leaves | Methanol | Very Good | 0.92 µg/ml IC50 | Plasmodium falciparum 3D7, W2 | No |
| Terminalia avicennioides | Combretaceae | [50] | Nigeria | Ns | Methanol, Water, Butanol, Ethyl Acetate | Moderate | 12.28–14.09 µg/ml IC50 | Plasmodium falciparum 3D7, K1 | Yes (SI ≥ 114; mouse heart-derived cells [NBMH]) |
| [52] | Burkina Faso | Leaves | Methanol | Very Good | 1.9 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| Terminalia glaucescens | Combretaceae | [39] | Ivory Coast | Stem, Leave | Water, Ethanol, Pentane | Very Good | 2.34–4.83 µg/ml IC50 | Plasmodium falciparum Fcm29, Fcb1, CQ-S (Nigerian) | No |
| Terminalia ivorensis | Combretaceae | [32] | Ghana | Stem Barks | Ethanol | Good | 6.949 µg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| [112] | Ghana | Leaves | Methanol | Good | 5.70 µg/ml IC50 | Plasmodium falciparum 3D7, W2 | No | ||
| Terminalia macroptera | Combretaceae | [27] | Burkina Faso | Root Barks | Water | Very Good | 1 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Terminalia mollis | Combretaceae | [92] | Rwanda | Root Barks | Methanol | Moderate | 11.7–26.3 µg/ml IC50 | Plasmodium falciparum 3D7, W2 | No |
| Terminalia spinosa | Combretaceae | [26] | Kenya | Stem Barks | Methanol | Good | 7.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Tetracera poggei Gilg | Dilleniaceae | [69] | DR Congo | Leaves | Petroleum Ether | Very Good | 1.7 µg/ml IC50 | Plasmodium falciparum | Nd |
| Tetrapleura tetraptera | Fabaceae | [33] | Gabon | Leaves | Dichloromethane | Moderate | 10.1–13.0 µg/ml IC50 | Plasmodium falciparum FCB, 3D7 | No |
| Thalia geniculata | Marantaceae | [65] | Benin | Roots | Methanol | Moderate | 2.83- > 20 µg/ml IC50 | Plasmodium falciparum 3D7 & K1 | Nd |
| Tinospora bakis | Menispermaceae | [34] | Sudan | Whole Plant | Petroleum Ether/Chloroform | Very Good | < 5 µg/ml IC50 | Plasmodium falciparum | Nd |
| Tithonia diversifolia | Asteraceae | [73] | S. Tome´ And Prı ´Ncipe | Aerial Parts | Petroleum Ether, Dichloromethane | Good | < 10 µg/ml IC50 | Plasmodium falciparum 3D7 And Dd2 | Nd |
| [92] | Rwanda | Flowers | Dichloromethane | Very Good | 1.0–1.1 µg/ml IC50 | Plasmodium falciparum 3D7, W2 | No | ||
| Toddalia asiatica | Rutaceae | [26] | Kenya | Root Barks | Methanol | Good | 6.82–13.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| [125] | Kenya | Root Barks | Dichloromethane + Methanol | Very Gooda | 9 – 100 ng/ml IC50 | Plasmodium falciparum | Nd | ||
| Trichilia emetica | Meliaceae | [121] | Mali | Leaves | Dichloromethane | Moderate | 11.9 µg/ml IC50 | Plasmodium falciparum 3D7 | Nd |
| [58] | Sudan | Leaves | Methanol | Good | 2.5–17.5 µg/ml IC50 | Plasmodium falciparum 3D7, Dd6 | Nd | ||
| [24] | Kenya | Stem Barks | Methanol | Moderate | 13.3 µg/ml C50 | Plasmodium falciparum D6, W2 | Nd | ||
| [25] | South Africa | Stem Barks | Acetone | Very Good | 3.29 µg/ml IC50 | Plasmodium falciparum UP1 (CQ-R) | Nd | ||
| [22] | South Africa | Leaves, Twigs | Dichloromethane/Methanol | Very Good | 3.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Triclisia dictyophylla | Menispermaceae | [40] | D.R. Congo | Leaves | Water | Good | 5.13 µg/ml IC50 | Plasmodium falciparum K1 | No |
| Tridax procumbens | Asteraceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Moderate | 17 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [26] | Kenya | Whole Plant | Methanol | Moderate | 15.4 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Triumfetta welwitschii var. hirsuta | Malvaceae | [22] | South Africa | Leaves | Dichloromethane/Methanol | Very Good | 3.6 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Turraea floribunda | Meliaceae | [22] | South Africa | Leaves | Dichloromethane/Methanol | Good | 8.8 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [26] | Kenya | Stem Barks | Methanol | Good | 5.5 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Turraea robusta | Meliaceae | [72] | Kenya | Root Barks | Methanol | Very Good | 2.4–3.5 µg/ml IC50 | Plasmodium falciparum K1, NF54 | Nd |
| [24] | Kenya | Stem Barks | Methanol | Good | 2.1–10.3 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Tylosema fassoglensis | Fabaceae | [30] | Kenya | Tubers | Dichloromethane | Very Good | 0.77–0.896 µg/ml IC50 | Plasmodium falciparum W2, D6 | Nd |
| Uapaca paludosa | Phyllanthaceae | [103] | Congo Brazzaville | Barks | Dichloromethane | Good | 8 µg/ml IC50 | Plasmodium falciparum Fcm29-Cameroon | Nd |
| Uvaria acuminata | Annonaceae | [26] | Kenya | Root Barks | Methanol | Good | 6.9–8.9 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Uvaria scheffleri | Annonaceae | [26] | Kenya | Leaves | Methanol | Good | 6.8 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Uvaria afzelii | Annonaceae | [48] | Ivory Coast | Roots | Pentane | Moderate | 9–22 µg/ml IC50 | Plasmodium falciparum FCM29, CQ-S (Nigerian) | No |
| Uvariastrum zenkeri | Annonaceae | [49] | Cameroon | Twigs | Ethanol | Very Good | 1.89 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Uvariodendron molundense | Annonaceae | [49] | Cameroon | Twigs | Methanol | Very Good | 4.79 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Uvariopsis congolana | Annonaceae | [55] | Cameroon | Stems | Ethanol, Water, Dichloromethane, Methanol, Hexane | Very Good | 4.47 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Vangueria infausta Burch. subsp. Infausta | Rubiaceae | [37] | South Africa | Roots | Dichloromethane | Very Good | 1.84 µg/ml IC50 | Plasmodium falciparum NF54 | Nd |
| Vepris lanceolata | Rutaceae | [20] | Kenya | Root Barks | Ethyl Acetate | Good | 7.0 µg/ml IC50 | Plasmodium falciparum K1 | Nd |
| Vernonia amygdalina | Asteraceae | [74] | S. Tome´ And Prı ´Ncipe | Leaves | Ethyl Acetate | Moderate | 10 µg/ml IC50 | Plasmodium falciparum 3D7 And Dd2 | Nd |
| [80] | Cameroon | Leaves | Dichloromethane | Moderate | 8.72–11.27 µg/ml IC50 | Plasmodium falciparum 3D7, DD2 | No | ||
| [126] | Nigeria | Leaves | Ethanol | Good | 9.83 µg/ml IC50 | Plasmodium falciparum 3D7, NF-54 | Yes (SI = 6.14; C-1008 kidney fibroblast | ||
| [26] | Kenya | Leaves | Methanol | Good | 4.9–7.2 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| [127] | Nigeria | Leaves | Ethanol | Moderate | 11.2 µg/ml IC50 | Plasmodium falciparum | Yes (LD50 = 1950 mg/kg; rat) | ||
| [69] | D.R. Congo | Leaves | Petroleum Ether | Very Good | 2.5 µg/ml IC50 | Plasmodium falciparum | Nd | ||
| Vernonia brachycalyx | Asteraceae | [104] | Kenya | Leaves | Dichloromethane/Ethyl Acetate | Good | 6.6—8.4 µg/ml IC50 | Plasmodium falciparum K39, V1/S | Nd |
| Vernonia cinerea | Asteraceae | [45] | Cambodia | Whole Plant | Dichloromethane | Moderate | 18.3 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Vernonia colorata | Asteraceae | [57] | Ivory Coast | Stems, Leaves | Water | Good | 2.35–9.38 µg/ml IC50 | Plasmodium falciparum Fcb1 & F32 | Nd |
| [54] | Zimbabwe | Leaves | Petrolether/Ethylacetate | Moderate | 12.1–17.8 µg/ml IC50 | Plasmodium falciparum Pow, Dd2 | Nd | ||
| [91] | Comoros | Roots | Dichloromethane | Very Good | 3 µg/ml IC50 | Plasmodium falciparum W2 | No | ||
| [22] | South Africa | Leaves | Dichloromethane/Methanol | Very Good | 4.7 µg/ml IC50 | Plasmodium falciparum D10 | Nd | ||
| Vernonia fastigiata | Asteraceae | [22] | South Africa | Leaves | Dichloromethane/Methanol | Moderate | 10 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Vernonia guineensis | Asteraceae | [128] | Cameroon | Leaves | Dichloromethane | Very Good | 1.635—1.823 µg/ml IC50 | Plasmodium falciparum | No |
| Vernonia lasiopus | Compositae | [12] | Kenya | Leaves | Chloroform, Ethylacetate, Methanol | Very Good | 1.0–3.2 µg/ml IC50 | Plasmodium falciparum K39 (CQ-S), ENT30, NF54, V1/S | Nd |
| [73] | Kenya | Root Barks | Dichloromethane | Very Good | 4.7–4.9 µg/ml IC50 | Plasmodium falciparum K1, NF54 | Nd | ||
| Vernonia myriantha | Asteraceae | [22] | South Africa | Leaves | Dichloromethane/Methanol | Very Good | 3 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Vernonia oligocephala | Asteraceae | [22] | South Africa | Leaves | Dichloromethane/Methanol | Very Good | 3.5 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Vismia guineensis | Hypericaceae | [48] | Ivory Coast | Leaves | Pentane | Moderate | 15–20 µg/ml IC50 | Plasmodium falciparum FCM29, CQ-S (Nigerian) | Nd |
| Warburgia ugandensis | Canellaceae | [72] | Kenya | Stem Barks | Dichloromethane | Very Good | 1.4–2.2 µg/ml IC50 | Plasmodium falciparum K1, NF54 | Nd |
| [24] | Kenya | Root Barks | Methanol | Good | 4.1–6.1 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Warburgia stuhlmannii | Canellaceae | [26] | Kenya | Stem Barks | Methanol | Very Good | 1.8–2.3 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Ximenia americana | Olacaceae | [57] | Ivory Coast | Stem, Leave | Water | Very Good | 0.6–2.6 µg/ml IC50 | Plasmodium falciparum Fcb1 & F32 | Nd |
| Xylopia aethiopica | Annonaceae | [98] | Cameroon | Stem Barks | Water | Moderatea | 17.8 µg/ml IC50 | Plasmodium falciparum W5 | Nd |
| [49] | Cameroon | Leaves | Methanol | Very Good | 3.75 µg/ml IC50 | Plasmodium falciparum W2 | Nd | ||
| Xylopia africana | Annonaceae | [49] | Cameroon | Stem Barks | Methanol | Very Good | 1.07 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Xylopia parviflora (A.Rich.)Benth.Oliv | Annonaceae | [37] | South Africa | Roots | Dichloromethane | Very Good | 2.19 µg/ml IC50 | Plasmodium falciparum NF54 | Nd |
| [49] | Cameroon | Leaves | Methanol | Very Good | 3.44 µg/ml IC50 | Plasmodium falciparum W2 | Nd | ||
| Xylopia phloiodora | Annonaceae | [98] | Cameroon | Stem Barks | Water | Moderatea | 17.9 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
| Xysmalobium undulatum | Apocynaceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Good | 6 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| Zanthoxylum chalybeum | Rutaceae | [137] | Kenya | Root Barks | Water | Good | 2.32–5.52 µg/ml IC50 | Plasmodium falciparum NF54, ENT30 | Nd |
| [77] | Uganda | Stem Barks | Ethyl Acetate | Very Good | 0.57–3.21 µg/ml IC50 | Plasmodium falciparum NF54 & FCR3 | Nd | ||
| [92] | Rwanda | Root Barks | Methanol | Very Good | 1.9–4.2 µg/ml IC50 | Plasmodium falciparum 3D7, W2 | No | ||
| [20] | Tanzania | Root Barks | Ethyl Acetate | Very Good | 4.2 µg/ml IC50 | Plasmodium falciparum K1 | Nd | ||
| [26] | Kenya | Root Barks | Methanol | Very Good | 2.9–3.7 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Zanthoxylum gilletii | Rutaceae | [43] | Ivory Coast | Stem Barks | Ethanol | Very Good | 2.8 µg/ml IC50 | Plasmodium falciparum FCB1 | Nd |
| Zanthoxylum heitzii | Rutaceae | [129] | Republic Of Congo | Barkss | Hexane | Very Gooda | 0.0089 µg/ml IC50 | Plasmodium falciparum, Plasmodium berghei | Nd |
| Zanthoxylum tsihanimposa | Rutaceae | [130] | Madagascar | Stem Barks | Dichloromethane + Methanol | Very Gooda | 98.4 µM IC50 | Plasmodium falciparum FCM29 | Nd |
| Zanthoxylum usambarense | Rutaceae | [24] | Kenya | Root Barks | Methanol | Good | 3.2–5.5 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Zea mays | Poaceae | [131] | Nigeria | Leaves | Ethanol, ethyl acetate | Good | 3.69—9.31 µg/ml IC50 | Plasmodium falciparum 3D7, INDO, Plasmodium berghei | Nd |
| Zehreria scabra | Cucurbitaceae | [22] | South Africa | Whole Plant | Dichloromethane/Methanol | Good | 5.6 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [26] | Kenya | Whole Plant | Methanol | Good | 9.8 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd | ||
| Ziziphus abyssica | Rhamnaceae | [24] | Kenya | Leaves | Methanol | Moderate | 17.5 µg/ml IC50 | Plasmodium falciparum D6, W2 | Nd |
| Ziziphus mucronata | Rhamnaceae | [22] | South Africa | Leaves | Dichloromethane | Moderate | 12 µg/ml IC50 | Plasmodium falciparum D10 | Nd |
| [25] | South Africa | Stem Barks | Acetone | Very Good | 4.13 µg/ml IC50 | Plasmodium falciparum UP1 (CQ-R) | Nd | ||
| Ziziphus cambodiana | Rhamnaceae | [45] | Cambodia | Stems | Dichloromethane | Moderate | 19.0 µg/ml IC50 | Plasmodium falciparum W2 | Nd |
Nd Not done, Ns Not specified, SI Selectivity index
aActivity determined using pure compounds isolated from plant
Table 2.
In vivo antimalarial activity of African medicinal plants
| Plant species | Plant family | Source | Country of study | Part of plant used | Extraction solvent | Antimalarial activity | Parasite suppression rate | Strain of Plasmodium tested | Toxicity (value; assay) |
|---|---|---|---|---|---|---|---|---|---|
| Acacia nilotica | Fabaceae | [132] | Nigeria | Roots | Water | Moderate | 79.5% at 400 mg/kg/day | Plasmodium berghei NK65 | No |
| [133] | Nigeria | Roots | Methanol | Very good | 62.59% at 150 mg/kg/day | Plasmodium berghei NK65 | No | ||
| Adansonia digitata | Malvaceae | [134] | Nigeria | Stem barks | Methanol | Moderatea | 90.18% at 400 mg/kg/day | Plasmodium berghei | Nd |
| [135] | Kenya | Stem barks | Ethanol | Very good | > 60% at 100 mg/kg/day | Plasmodium berghei | No | ||
| [135] | Kenya | Stem barks | Water | Very good | 60.47% at 100 mg/kg/day | Plasmodium berghei | No | ||
| Ageratum conyzoides | Asteraceae | [136] | Nigeria | Leaves | Water | Moderate | 89.87% at 400 mg/kg/day | Plasmodium berghei NK65 | Nd |
| Albizia gummifera | Fabaceae | [137] | Kenya | Root barks | Methanol | Very gooda | 72.9% at 20 mg/kg.day | Plasmodium falciparum NF54 and ENT36 | Nd |
| Allophylus africanus | Sapindaceae | [138] | Nigeria | Stems, roots | Ns | Very good | 92.82–97.81 at 50 mg/kg/day | Plasmodium berghei NK-65 | Nd |
| Aloe pulcherrima | Xanthorrhoeaceae | [139] | Ethiopia | Leaves | Methanol | Gooda | 56.2 at 200 mg/kg/day | Plasmodium berghei | No |
| Anthocleista djalonensis | Gentianaceae | [140] | Nigeria | Roots | Chloroform, ethyl acetate, methanol | Moderate | 64.81–87.66% at 500 mg/kg/day | Plasmodium beghei ANKA | No |
| [140] | Nigeria | Roots | Ethanol, chloroform, ethyl acetate, methanol | Moderate | 67.92% at 500 mg/kg/day | Plasmodium berghei ANKA | No | ||
| Artemisia macivarae | Asteraceae | [141] | Nigeria | Whole plant | Chloroform | Very good | 80% at 100 mg/kg | Plasmodium berghei | Nd |
| Aspilia africana | Asteraceae | [142] | Nigeria | Leaves | Ethanol | Moderate | 92.23% at 400 mg/kg/day | Plasmodium berhhei NK65 | No |
| Azadirachta indica | Meliaceae | [143] | Kenya | Leaves | Methanol | Good | 83.48% at 250 mg/kg/day | Plasmodium falciparum D6 and W2 | No |
| [144] | Cameroon | Leaves | Ethanol | Moderate | 69.28% at 300 mg/kg/day | Plasmodium berghei NK65 | No | ||
| [145] | Nigeria | Leaves | Methanol | Very good | 56 – 87% at 50 mg/kg/day | Plasmodium berghei ANKA | No | ||
| Balanites rotundifolia | Zygophyllaceae | [146] | Ethiopia | Leaves | Methanol | Moderate | 67% at 400 mg/dl | Plasmodium berghei | No |
| Blighia sapida | Sapindaceae | [147] | Nigeria | Leaves | Ethanol | Good | 57% at 200 mg/kg/day | Plasmodium berghei ANKA | No |
| Bombax buonopozense | Malvaceae | [148] | Nigeria | Root barks | Water | Good | 93% at 200 mg/kg/day | Plasmodium berghei NK65 | Nd |
| Brassica nigra | Brassicaceae | [149] | Ethiopia | Seeds | Methanol | Moderate | 53.13% at 400 mg/kg/day | Plasmodium berghei ANKA | Nd |
| Calpurnia aurea | Fabaceae | [150] | Ethiopia | Leaves | Hydroalcohol | Very good | 51.15% at 60 mg/kg | Plasmodium berghei | No |
| Carica papaya | Caricaceae | [151] | Nigeria | Leaves | Ethanol | Good | 59.29% at 200 mg/kg | Plasmodim berghei NK65 | Nd |
| Senna occidentalis | Fabaceae | [152] | D.R. Congo | Root barks | Ethanol | Good | 68% at 200 mg/kg | Plasmodium berghei ANKA | No |
| Cassia sieberiana | Fabaceae | [153] | Nigeria | Stems | Ethanol | Good | 63.9% at 300 g/kg/day | Plasmodium berghei NK65 | No |
| Cassia singueana | Fabaceae | [154] | Nigeria | Root barks | Methanol | Good | 79.06% at 200 mg/kg/day | Plasmodium berghei | Yes (LD50 = 847 mg/kg; mice) |
| Chrozophora senegalensis | Euphorbiaceae | [155] | Nigeria | Whole plant | Methanol | Very good | 51.8% at 75 mg/kg/day | Plasmodium berghei | Nd |
| Chrysophyllum albidum | Sapotaceae | [156] | Nigeria | Seeds, pulp juice | Ethanol | Moderate | 72.97% at 500 mg/kg | Plasmodium berghei | No |
| Clausena anisota | Rutaceae | [157] | Nigeria | Leaves | Ethanol | Very good | 82.02% at 78 mg/kg/day | Plasmodium berghei | Yes (LD50 = 393.7 mg/kg; albino mice) |
| Combretum molle | Combretaceae | [158] | Ethiopia | Seeds | Methanol | Good | 63.5% at 250 mg/kg/day | Plasmodium berghei ANKA | Nd |
| Commiphora africana | Burseraceae | [159] | Tanzania | Stem barks | Dichloromethane | Moderate | 64.24% at 400 mg/kg/day | Plasmodium falciparum (D6, Dd2), Plasmodium berghei | No |
| Crossopteryx febrifuga | Rubiaceae | [160] | Nigeria | Stem barks | Ethanol | Good | 63.65% at 200 mg/kg/day | Plasmodium berghei var. ANKA | Nd |
| Croton macrostachyus | Euphorbiaceae | [161] | Kenya | Stem barks | Ethyl acetate | Moderate | 82% at 500 mg/kg/day | Plasmodium berghei ANKA | Nd |
| Cryptolepis sanguinolenta | Apocynaceae | [162] | Congo | Root barks | Ethanol | Moderate | 75.07% at 400 mg/kg/day | Plasmodium falciparum, Plasmodium berghei berghei | Nd |
| [84] | Ghana | Roots | Hexane, ethanol, dichloromethane | Very good* | > 80% at 2.5 mg/kg/day | Plasmodium vinckei petteri, Plasmodium berghei ANKA | Nd | ||
| Cucumis metuliferus | Cucurbitaceae | [163] | Tanzania | Leaves | Chloroform | Moderate | 70.69% at 600 mg/kg/day | Plasmodium berghei ANKA | Nd |
| Dichrostachys cinerea | Fabaceae | [159] | Tanzania | Stem barks | Methanol | Moderate | 53.12% at 400 mg/kg/day | Plasmodium falciparum (D6, Dd2), Plasmodium berghei | No |
| Dodonaea angustifolia | Sapindaceae | [164] | Ethiopia | Roots | N-butanol | Moderate | 55.8% at 400 mg/kg/day | Plasmodium berghei | Nd |
| Enantia chlorantha Oliv | Annonaceae | [165] | Nigeria | Stem barks | Ethanol | Moderate | 75.23% at 500 mg/kg | Plasmodium berghei NK-65 | Nd |
| Erigeron floribundus | Asteraceae | [144] | Cameroon | Whole plant | Ethanol | Good | 62.4% at 240 mg/kg/day | Plasmodium berghei NK65 | No |
| Euphorbia cordifolia | Euphorbiaceae | [166] | Cameroon | Whole plant | Aqueous | Very good | 94.70% at 200 mg/kg/day | Plasmodium berghei | No |
| Euphorbia hirta L | Euphorbiaceae | [162] | Congo | Whole plant | Ethanol | Moderate | 69.44% at 400 mg/kg/day | Plasmodium falciparum, Plasmodium berghei berghei | Nd |
| Faidherbia albida | Fabaceae | [167] | Nigeria | Stem barks | Ethanol | Moderate | 89.5 at 400 mg/kg/day | Plasmodium berghei NK65 | Nd |
| Grewia plagiophylla | Malvaceae | [143] | Kenya | Leaves | Methanol | Moderate | 77.9 at 250 mg/kg/day | Plasmodium falciparum D6 and W2 | Nd |
| Grewia trichocarpa | Malvaceae | [168] | Kenya | Root | Water | Good | 35.8% at 10 mg/kg/day | Plasmodium berghei | Yes (LD50 = 545.8 µg/ml; brine shrimp) |
| Garcinia kola | Clusiaceae | [169] | Nigeria | Seeds | Petroleum ether | Very good* | 93% at 200 mg/kg/day | Plasmodium berghei | Nd |
| Hippocratea africana | Celastraceae | [170] | Nigeria | Nd | Ethanol | Moderate | 90.9% at 600 mg/kg/day | Plasmodium berghei berghei | Yes (LD50 = 2449 mg/kg; mice) |
| Hoslundia opposita | Lamiaceae | [143] | Kenya | Leaves | Methanol | Moderate | 79.67% at 250 mg/kg/day | Plasmodium falciparum D6 and W2 | Yes (CC50 = 37 µg/ml; Vero E6 cells) |
| Icacina senegalensis | Icacinaceae | [171] | Nigeria | Leaves | Methanol | Very good | 80% at 100 mg/kg/day | Plasmodium berghei | Yes (LD50 > 2000 mg/kg; mice) |
| Indigofera spicata | Fabaceae | [172] | Ethiopia | Roots | Methanol | Moderate | 53.42% at 600 mg/kg/day | Plasmodium berghei ANKA | Nd |
| Lannea schweinfurthii | Anacardiaceae | [143] | Kenya | Leaves | Methanol | Moderate | 83.48% at 250 mg/kg/day | Plasmodium falciparum D6 and W2 | Yes (CC50 = 76 µg/ml; Vero E6 cells) |
| Lippia kituiensis | Verbenaceae | [163] | Tanzania | Leaves | Ethyl acetate | Moderate | 70.14% at 600 mg/kg/day | Plasmodium berghei ANKA | Nd |
| Lophira lanceolata | Ochnaceae | [173] | Nigeria | Leaves | Methanol | Moderate | 80% at 400 mg/kg/day | Plasmodium berghei | No |
| Maerua crassifolia | Capparaceae | [174] | Nigeria | Leaves | Methanol | Moderate | 86% at 400 mg/kg/day | Plasmodium berghei NK65 | No |
| Maytenus senegalensis | Celastraceae | [175] | Tanzania | Root barks | Ethanol | Very good | 98.1% at 100 mg/kg/day | Plasmodium berghei | No |
| Morinda morindoides | Rubiaceae | [152] | D.R. Congo | Leaves | Dichloromethane | Good | 74% at 200 mg/kg/day | Plasmodium berghei ANKA | No |
| Mucuna pruriens | Fabaceae | [176] | Nigeria | Leaves | Water | Good | 71.75% at 270 mg/kg/day | Plasmodium berghei NK65 | No |
| Nauclea latifolia | Rubiaceae | [177] | Nigeria | Leaves | Ethanol | Moderate | 60.63% at 500 mg/kg/day | Plasmodium berghei | No |
| [165] | Nigeria | Roots | Ethanol | Moderate | 71.15% at 500 mg/kg/day | Plasmodium berghei NK-65 | Nd | ||
| Oldenlandia affinis | Rubiaceae | [178] | Nigeria | Aerial parts | Methanol, water, dichloromethane | Moderate | 75% at 400 mg/kg/day | Plasmodium berghei | No |
| Peschiera fuchsiaefolia | Apocynaceae | [179] | Madagascar | Stem barks | Ns | Good* | 43.4% at 10 mg/kg/day | Plasmodium yoelii N67, Plasmodium falciparum FMC29 | Nd |
| Phyllanthus amarus | Phyllanthaceae | [180] | Nigeria | Whole plant | Water and ethanol | Good | 79% at 1600 mg/kg/day | Plasmodium yoelii | Nd |
| Phyllanthus niruri | Phyllanthaceae | [152] | D.R. Congo | Whole plant | Ethanol | Good | 73% at 200 mg/kg/day | Plasmodium berghei ANKA | No |
| [181] | Nigeria | Aerial parts | Methanol/chloroform | Very good | 90.48% at 100 mg/kg/day | Plasmodium berghei berghei NK 65 | Nd | ||
| Phytolacca dodecandra | Phytolaccaceae | [182] | Ethiopia | Leaves | Methanol | Moderate | 55.24% at 400 mg/kg/day | Plasmodium berghei | Nd |
| Picralima nitida | Apocynaceae | [183] | Nigeria | Seeds | Ethanol | Good | 73% at 115 mg/kg/day | Plasmodium berghei berghei | Yes (LD50 = 87.29 µg/ml; albino mice) |
| Piliostigma thonningii | Fabaceae | [184] | Nigeria | Leaves | Ethanol | Moderate | 91% at 400 mg/kg/day | Plasmodium berghei NK65 | No |
| Premna chrysoclada | Lamiaceae | [143] | Kenya | Leaves | Methanol | Good | 65.08% at 250 mg/kg/day | Plasmodium falciparum D6 and W2 | Nd |
| Pseudocedrela kotschyi | Meliaceae | [185] | Nigeria | Leaves | Ethanol | Moderate | 90% at 400 mg/kg/day | Plasmodium berghei (NK65 | No, |
| Rhus natalensis | Anacardiaceae | [143] | Kenya | Leaves | Methanol | Moderate | 82.7% at 250 mg/kg/day | Plasmodium falciparum D6 and W2 | Nd |
| Salacia nitida | Celastraceae | [165] | Nigeria | Roots | Ethanol | Moderate | 71.15% at 250 mg/kg/day | Plasmodium berghei NK-65 | Nd |
| Stachytarpheta cayennensis | Verbenaceae | [186] | Nigeria | Leaves | Ethanol | Good | 78.2% at 270 mg/kg/day | Plasmodium berghei berghei | Yes (LD50 = 938.08 mg/kg; albino mice) |
| Telfairia occidentalis | Cucurbitaceae | [187] | Nigeria | Leaves | Water | Good | 72.17% at 200 mg/kg/day | Plasmodium berghei ANKA | No |
| Tithonia diversifolia | Asteraceae | [160] | Nigeria | Aerial parts | Ethanol | Good | 74.97% at 200 mg/kg/day | Plasmodium berghei var. ANKA I | Nd |
| Toddalia asiatica | Rutaceae | [188] | Kenya | Root barks | Methanol | Moderate | 59.3% at 500 mg/kg/day | Plasmodium berghei NK66 | Nd |
| Trema orientalis | Cannabaceae | [189] | Nigeria | Stem barks | Methanol | Good | 70% at 200 mg/kg/day | Plasmodium berghei | Nd |
| Trichilia megalantha | Meliaceae | [190] | Nigeria | Stem barks | Methanol, chloroform | Good | 89.1–100% at 200 mg/kg/day | Plasmodium berghei berghei ANKA | Nd |
| Triphyophyllum peltatum | Dioncophyllaceae | [191] | Ivory Coast | Roots, stem barks | Dichloromethane | Very good* | 99% at 50 mg/kg/day | Plasmodium berghei ANKA CRS | Nd |
| Uvaria acuminata | Annonaceae | [143] | Kenya | Roots | Methanol | Good | 27.0% at 250 mg/kg/day | Plasmodium falciparum D6 and W2 | Nd |
| Uvaria chamae P. Beauv | Annonaceae | [170] | Nigeria | Nd | Ethanol | Moderate | 72.2% at 600 mg/kg/day | Plasmodium berghei berghei | Yes (LD50 = 3464 mg/kg; mice) |
| Verbena hastata | Verbenaceae | [192] | Nigeria | Leaves | Ethanol | Moderate | 70% at 400 mg/kg/day | Plasmodium berghei | No |
| Vernonia amygdalina | Asteraceae | [193] | Uganda | Leaves | Water | Good | 73% at 200 mg/kg/day | Plasmodium berghei | No |
| [194] | Nigeria | Leaves | Water | Good | 50.78—62.66% at 125 mg/kg/day | Plasmodium berghei ANKA | Nd | ||
| [195] | Botswana | Leaves and root barks | Ethanol | Moderate | 67% at 500 mg/kg/day | Plasmodium berghei | Nd | ||
| Vernonia lasiopus | Asteraceae | [188] | Kenya | Root barks | Methanol | Moderate | 59.3% at 500 mg/kg/day | Plasmodium berghei NK67 | Nd |
| Withania somnifera | Solanaceae | [196] | Ethiopia | Leaves | Methanol | Moderate | 57% at 300 mg/kg/day | Plasmodium berghei ANKA | Nd |
| Xylopia aethiopica | Annonaceae | [141] | Nigeria | Fruits | Chloroform | Very good | 60% at 100 mg/kg/day | Plasmodium berghei | Nd |
| Artemisia abyssinica | Asteraceae | [197] | Ethiopia | Aerial parts | Hydroalcohol | Good | 64.7% at 200 mg/kg/day | Plasmodium berghei | Nd |
| Rotheca myricoides | Lamiaceae | [198] | Ethiopia | Leaves | Methanol | Good | 54.14% at 200 mg/kg/day | Plasmodium berghei | No |
| Dodonaea angustifolia | Sapindaceae | [198] | Ethiopia | Roots | Methanol | Good | 57.74% at 200 mg/kg/day | Plasmodium berghei | No |
| Clutia abyssinica | Peraceae | [199] | Kenya | Leaves | Methanol | Moderate | 40.45% at 100 mg/kg/day | Plasmodium falciparum, Plasmodium berghei ANKA | No |
| Pittosporum viridiflorum | Pittosporaceae | [199] | Kenya | Leaves | Methanol | Moderate | 54.77% at 100 mg/kg/day | Plasmodium falciparum D6 &W2, Plasmodium berghei ANKA | Yes (SI = 2.51; Vero E6 cells) |
Nd Not done, Ns Not specified, SI Selectivity index
aActivity determined using pure compounds isolated from plant
Table 3.
In vitro and in vivo studies on African medicinal plants
| Plant species | Plant family | Source | Country of study | Part of plant used | Extraction solvent | Overall activity | In vitro | In vivo | IC50 or ED50 or LD50 | Strain of Plasmodium tested | parasite suppression rate | Toxicity (value; assay) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphaeranthus suaveolens | Compositae | [199] | Kenya | Whole plant | Methanol | Moderate | Moderate | In active | 7.93–56.73 µg/ml IC50 | Plasmodium falciparum D6 and W2, Plasmodium berghei ANKA | 46.74% at 100 mg/kg/day | No |
| Abutilon grandiflorum | Malvaceae | [200] | Tanzania | Roots | Ethyl acetate | Good | Moderate | Very good | 9–14 µg/mL IC50 | Plasmodium falciparum HB3 and FCB, Plasmodium vinckei vinckei | 83–87% at 20 ug/ml/day | Yes (IC50 = 36 µg/ml; human colon carcinoma cell line [HT29]) |
| Alchornea laxiflora | Euphorbiaceae | [131] | Nigeria | Roots | Ethyl acetate, dichloromethane | Good | Inactive | Very good | 38.44—40.17 µg/ml IC50 | Plasmodium falciparum 3Dè, INDO, Plasmodium berghei | 65.73% at 150 mg/kg/day | Yes (LD50 = 748.33 mg/kg; HeLa cells) |
| Annona senegalensis | Annonaceae | [201] | Nigeria | Leaves | Methanol | Moderate | In active | Very good | 28.8 µg/ml IC50 | Plasmodium berghei | > 57% at 100 mg/kg/day | No |
| Boscia angustifolia | Capparaceae | [199] | Kenya | Stem barks | Methanol | Moderate | Moderate | Very good | 7.43–35.93 µg/ml IC50 | Plasmodium falciparum D6 &W2, Plasmodium berghei ANKA | 60.12% at 100 mg/kg/day | No |
| Chrozophora senegalensis | Euphorbiaceae | [64] | Senegal | Leaves | Water | Very good | Very good | Very good | 1.6–1.9 µg/ml IC50 | Plasmodium falciparum FcM29, FcB1, Plasmodium vinckei petteri | 65% at 10 mg/kg/day | No |
| Clerodendrum eriophyllum | Lamiaceae | [199] | Kenya | Root barks | Methanol | Moderate | Good | Very good | 9.51–10.56 µg/ml IC50 | Plasmodium falciparum D6 & W2, Plasmodium berghei ANKA | 90.13% at 100 mg/kg/day | No |
| Cocos nucifera | Arecaceae | [202] | Nigeria | Husk | Ethyl acetate | Moderate | Moderate | Very good | 10.94 µg/ml IC50 | Plasmodium falciparum W2, Plasmodium berghei NK65 | 98.6% at 125 mg/kg/day | Nd |
| Commiphora africana | Burseraceae | [159] | Tanzania | Stem barks | Dichloromethane | Moderate | Very good | Moderate | 4.54 µg/ml IC50 | Plasmodium falciparum D6, Dd2, Plasmodium berghei | 64.24% at 400 mg/kg/day | No |
| Ficus thonningii | Moraceae | [203] | Nigeria | Whole plant | Hexane | Moderate | Good | Moderate | 2.7–10.4 µg/ml IC50 | Plasmodium falciparum NF54, K1, Plasmodium berghei NK65 | 84.5% at 500 mg/kg/day | No |
| Flueggea virosa | Phyllanthaceae | [199] | Kenya | Leaves | Methanol | Very good | Very good | Very good | 2.28–3.64 µg/ml IC50 | Plasmodium falciparum D6 and W2, Plasmodium berghei ANKA | 70.91% at 100 mg/kg/day | No |
| Fuerstia africana | Lamiaceae | [199] | Kenya | Whole plant | Methanol | Very good | Very good | Very good | 0.98–2.40 µg//ml IC50 | Plasmodium falciparum D6 and W2, Plasmodium berghei ANKA | 61.85% at 100 mg/kg/day | No |
| Harungana madagascariensis | Hypericaceae | [199] | Kenya | Leaves | Water | Moderate | Inactive | Very good | 39.07–43.7 µg/ml IC50 | Plasmodium falciparum D6 and W2, Plasmodium berghei ANKA | 88.04% at 100 mg/kg/day | No |
| [204] | Nigeria | Stem barks | Ethanol | Very good | Very good | Inactive | 0.052—0.517 μg/ml IC50 | Plasmodium yoelii nigeriensis N67, Plasmodium falciparum | 28.6–44.8% | Nd | ||
| Lannea schweinfurthii | Anacardiaceae | [205] | Kenya | Stem barks | Methanol | Moderate | Moderate | Very good | 11.38–36.26 µg/ml IC50 | Plasmodium falciparum D6, W2, Plasmodium berghei | 91.37% at 100 mg/kg/day | Yes (SI = 6.21–19.79; Vero cells) |
| Lophira alata | Ochnaceae | [203] | Nigeria | Whole plant | Hexane | Good | Very good | Moderate | 2.5 µg/ml IC50 | Plasmodium falciparum NF54, K1, Plasmodium berghei NK65 | 74.45% at 500 mg/kg/day | No |
| Ludwigia erecta | Onagraceae | [199] | Kenya | Whole plant | Water | Very good | Very good | In active | 0.93–1.61 µg/ml IC50 | Plasmodium falciparum D6 & W2, Plasmodium berghei ANKA | 49.64% at 100 mg/kg/day | No |
| Maytenus putterlickioides | Celastraceae | [199] | Kenya | Root barks | Methanol | Good | Good | Very good | 4.41–10.26 µg/ml IC50 | Plasmodium falciparum D6 and W2, Plasmodium berghei ANKA | 78.66% at 100 mg/kg/day | No |
| Maytenus undata | Celastraceae | [199] | Kenya | Leaves | Methanol | Good | Good | Very good | 7.4–9.89 µg/ml IC50 | Plasmodium falciparum D6 and W2, Plasmodium berghei ANKA | 76.29% at 100 mg/kg/day | No |
| Mimusops caffra | Sapotaceae | [206] | South Africa | Leaves | Dichloromethane | Good | Very good | Moderate | 2.14 µg/ml IC50 | Plasmodium falciparum D10, Plasmodium berghei | 94.01% at 400 mg/kg/day | Nd |
| Schkuhria pinnata | Compositae | [199] | Kenya | Whole plant | Methanol | Good | Good | In actice | 1.3–6.83 µg/ml IC50 | Plasmodium falciparum D6 & W2, Plasmodium berghei ANKA | 49.9% at 100 mg/kg/day | No |
| Sclerocarya birrea | Anacardiaceae | [205] | Kenya | Stem barks | Methanol | Moderate | Moderate | Very good | 5.91–24.96 µg/ml IC50 | Plasmodium falciparum D6, W2, Plasmodium berghei | 63.49% at 100 mg/kg/day | No |
| Toddalia asiatica | Rutaceae | [117] | Kenya | Fruits | Ethyl acetate | Very good | Very good | Moderate | 1.87 μg/ml IC50 | Plasmodium falciparum W2 & D6, Plasmodium berghei | 81.34% at 500 mg/kg/day | No |
| Turraea robusta | Meliaceae | [205] | Kenya | Root barks | Methanol | Good | Good | Very good | 2.09–10.32 µg/ml IC50 | Plasmodium falciparum D6, W2, Plasmodium berghei | 78.2% at 100 mg/kg/day | Yes (SI = 2.36–11.67; Vero cells) |
| Uapaca nitida | Phyllanthaceae | [207] | Tanzania | Root barks | Ethanol | Moderate* | Inactive | Inactive | 19.6—25.9 µg/mL IC50 | Plasmodium falciparum K1, T9-96 & Plasmodium berghei | poor | No |
| Vernonia ambigua | Asteraceae | [208] | Nigeria | Ns | Water | Very good | Inactive | Very good | 31.26–50 µg/ml IC50 | Plasmodium berghei, Plasmodium falciparum | 60% at 100 mg/kg/day | No |
| [209] | Republic of Congo | Leaves | Methanol | Moderate | Very good | Moderate | 3.58 µg/ml IC50 | Plasmodium falciparum. Plasmodium yoelii | 61.28% at 500 mg/kg/day | No | ||
| Warburgia stuhlmannii | Camellaceae | [199] | Kenya | Stem barks | Water | Very good | Very good | Very good | 1.81–2.33 µg/ml IC50 | Plasmodium falciparum D6 and W2, Plasmodium berghei ANKA | 84.95% at 100 mg/kg/day | No |
| Azadirachta indica | Meliaceae | [143] | Kenya | Leaves | Methanol | Good | Good | Good | 6.24–7.53 µg/ml IC50 | Plasmodium falciparum D6 and W2 | 83.48% at 250 mg/kg/day | No |
| Dichrostachys cinerea | Fabaceae | [159] | Tanzania | Stem barks | Methanol | Moderate | Good | Moderate | 2.37–11.92 µg/ml IC50 | Plasmodium falciparum D6, Dd2, Plasmodium berghei | 53.12% at 400 mg/kg/day | No |
| Grewia plagiophylla | Malvaceae | [143] | Kenya | Leaves | Methanol | Moderate | Moderate | Good | 13.28–34.2 µg/ml IC50 | Plasmodium falciparum D6 and W2 | 77.9% at 250 mg/kg/day | Nd |
| Hoslundia opposita | Lamiaceae | [143] | Kenya | Leaves | Methanol | Moderate | Good | Good | 12.8–13.22 µg/ml IC50 | Plasmodium falciparum D6 and W2 | 79.67% at 250 mg/kg/day | Yes (SI = 0.58; Vero E6 cells) |
| Lannea schweinfurthii | Anacardiaceae | [143] | Kenya | Leaves | Methanol | Moderate | Inactive | Good | 38.87–54.15 µg/ml IC50 | Plasmodium falciparum D6 and W2 | 83.48% at 250 mg/kg/day | Yes (SI = 1.4; Vero E6 cells) |
| Premna chrysoclada | Lamiaceae | [143] | Kenya | Leaves | Methanol | Good | Good | Good | 7.75–9.02 µg/ml IC50 | Plasmodium falciparum D6 and W2 | 65.08% at 250 mg/kg/day | Nd |
| Rhus natalensis | Anacardiaceae | [143] | Kenya | Leaves | Methanol | Moderate | Inactive | Good | 43.93–51.2 µg/ml IC50 | Plasmodium falciparum D6 and W2 | 82.7% at 250 mg/kg/day | Nd |
| Triphyophyllum peltatum | Dioncophyllaceae | [191] | Ivory coast | Roots, stem barks | Dichloromethane | Very good* | Very good | Very good | 1.90 mg/kg for Dioncophylline C and 10.71 mg/kg for dioncophylline A | Plasmodium berghei ANKA CRS | 99% at 50 mg/kg/day | Nd |
| Uvaria acuminata | Annonaceae | [143] | Kenya | Roots | Methanol | Good | Good | In active | 6.90–8.89 µg/ml IC50 | Plasmodium falciparum D6 and W2 | 27.0% at 250 mg/kg/day | Nd |
Nd Not done, Ns Not specified, SI Selectivity index
aActivity determined using pure compounds isolated from plant
Table 4.
Clinical trial on African medicinal plants
| Plant species | Plant family | Source | Country of study | Part of plant used | Extraction solvent | Crude extract? | Antimalarial activity | Parasite suppression rate | Strain of Plasmodium tested | Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| Cochlospermum planchonii | Bixaceae | [210] | Burkina Faso | Roots | Ns | Yes | Moderate | 52 at 600 ml/day | Plasmodium falciparum | No |
Nd Not done, Ns Not specified
Fig. 2.
Distribution of the research articles on the antiplasmodial activity of indigenous plants according to African countries
Family and species distribution of plants evaluated
From 722 studies, the most frequent plant families studied included Fabaceae 47 (6.5%), Euphorbiaceae 45 (6.2%), Annonaceae 37 (5.1%), Rubiaceae 37 (5.1%), Rutaceae 37 (5.1%), Meliaceae 30 (4.2%), and Lamiaceae 12 (1.7%). Five hundred and two (502) plant species were investigated in this study. Of them, the most investigated were: Azadirachta indica, Zanthoxylum chalybeum, Picrilima nitida, and Nauclea latifolia. The most frequent parts of the plants tested were the leaves, roots, root barkss, stems, and the whole plant. A majority of the studies used the crude extracts of the plants compared to pure compounds (95.7% vs. 4.3%). In descending order, methanol 322 (44.7%), dichloromethane 207 (28.7%), ethanol 103 (14.3%), water 85 (11.7%) and ethyl acetate 62 (8.6%) were the most frequent extraction solvent used.
In vitro and in vivo activities of the plants evaluated
Overall, 248 (34.3%) of the studies reported activity that was very good (IC50 values < 5 µg/ml or suppression rate of ≥ 50% at 100 mg/kg body weight/day), 241 (33.4%) reported good activity and 233 (32.3%) reported moderate activity. For the in vitro studies, a majority 228 (38.6%) reported very good activity; 206 (34.9%) reported good activity and 187 (31.6%) reported moderate activity. Meanwhile for the in vivo studies, a majority 19 (21.1%) reported moderate activity, 16 (17.8%) reported very good activity and 13 (14.4%) reported good activity. For studies reporting both the in vitro and in vivo activity, a majority of 17 (42.5%) reported only moderate activity, 13 (32.5%) studies reported very good activity and 10 (25.0%) reported good activity. Among the plants with very good activity, only one species demonstrated very good activity both in vitro and in vivo (Table 3).
Among the studies, the most frequent plant species demonstrating very good antiplasmodial activity were: Alchornea cordifolia [3/3, 100%], Flueggea virosa [3/3, 100%], Cryptolepis sanguinolenta [¾, 75%], Zanthoxylum chalbeum [4/5, 80%] and Maytenus senegalensis [3/6, 50%]. Plant families with the most active species include Rutaceae [13/25, 52.0%], Apocynaceae [13/26, 50%], Celastraceae [7/15, 46.7%], Annonaceae [17/37, 45.9%], Euphorbiaceae [21/48, 43.8], Combretaceae [7/16, 43.8%], Fabaceae [18/47, 38.3%], Lamiaceae [8/23, 34.8%], Asteraceae [23/69, 33.3%], and Rubiaceae [8/37, 21.6%]. The fractions are derived from the count of studies reporting very good antiplasmodial activity (numerator) divided by the total number of studies that assessed the activity of that plant species (denominator).
Azadirachta indica and Vernonia amygdalina were the most frequently reported inactive species (Additional file 1: Table S1). Furthermore, Fabaceae, Rubiaceae, Euphorbiaceae, and Asteraceae were the plant families containing the most frequently reported inactive plants. A majority of 95.7% (691/722) of the studies used the crude extract of the plants. The antiplasmodial and/or anti-malarial activity was significantly higher (p = 0.044) in studies using pure compounds compared to those using crude preparations.
Toxicity of plants evaluated for their antiplasmodial and anti-malarial activity
Out of the 198 plants evaluated in toxicity assays, 52 (26.3%) were found to demonstrate some degree of toxicity. The most frequently reported plants with toxicity were Azadirachta indica and Vernonia amygdalina. Plant families harboring the most toxic species were Lamiaceae, Anacardiaceae, Moraceae, Meliaceae, Asteraceae, and Fabaceae. Approximately 33% of the plants tested demonstrated some toxicity in vitro and 26.7% had some degree of toxicity in vivo. Among plants with very good, good, and moderate antiplasmodial activity, 17.8%, 28.3%, and 35.4% had some degree of toxicity, respectively. The leaf was the plant part with the most frequently reported toxicity. Albino mice and Vero E6 cells were the most commonly used assays for the assessment of the toxicity of the plants.
Discussion
Resistance to the frontline anti-malarial drugs is increasing and is now a global concern. With this rising rate of resistance, there is a need to accelerate research into the discovery and development of new anti-malarial drugs. Unfortunately, from this study, it is evident that the progress into the discovery of a new anti-malarial drug in Africa is slothful. Despite a considerable number of plant species that have demonstrated significant antiplasmodial activity in vitro, fewer plants have been evaluated in vivo and only one clinical trial with Cochlospermum planchonii (Bixaceae) has been conducted so far. This reinforces the need for basic and clinical research in the region. Van Wyk [213] had also arrived at the same conclusion.
This review revealed research articles from 31 African countries. Most of the articles were from Nigeria. This is suggestive that Nigeria is leading the podium in research on anti-malarial drug discovery and development, deservedly so, because she is probably the most affected country in the world. It is noteworthy that South Africa which is generally more technologically advanced than Nigeria had very few (8) articles. The African region is the most affected in the world recording the greatest number of cases and malaria attributed deaths. However, the distribution of malaria in Africa is not even, with sub-Saharan Africa harboring disproportionately the greatest number of cases. This is suggestive that research to identify new anti-malarial drugs may be related to the burden of the disease, thus the government policy to control the disease. There is, therefore, the need for policy-driven research into new anti-malarial all across the African region. In this review, IC50 values of < 20 µg/ml were considered as the cutoff of significant anti-malarial activity. This cutoff is considered the minimum to qualify as a first-pass “hit” in anti-malarial drugs screening [214]. Five hundred and two (502) plant species from 169 families were observed to have moderate to very good anti-malarial activity. The most investigated plant families were Euphorbiaceae, Fabaceae, Rubiaceae, and Annonaceae. However, the plant families containing the most active plants were Apocynaceae, Celestraceae, and Rutaceae. This finding suggests that more emphasis should be given to plants in these families for anti-malarial drug discovery. Besides, the most investigated plant species were Azadirachta indica, Nauclea latifolia, Picralima nitida, and Zanthoxylum chalybeum. Alchornea cordifolia, Flueggea virosa, Crytolepis sanguinolenta, and Zanthoxylum chalybeum were the only plant species with consistently very good antiplasmodial and anti-malarial activities between studies. This is very surprising that no clinical trial using any of these plants has been conducted. Further studies on these plant species should be performed.
This study revealed that overall, a majority of the plants investigated had very good antiplasmodial activity in vitro. That activity decreases as you move to in vivo in most studies, with a majority of plants demonstrating only moderate activity. For example, Gathirwa et al. [146] showed that the activity of Uvaria acuminate decreased from good activity in vitro to inactive in vivo. However, a few studies show that plant activity could also increase from in vitro to in vivo. For example, Ngbolua et al. [211] showed that the activity of Vernonia ambigua increased from in vitro to in vivo analysis. Other examples include studies by Muthaura et al. [20] using Boscia angustifolia, Kweyamba et al. [162] using Commiphora Africana, and Ajaiyeoba et al. [204] using Annona senegalensis. This suggests that plants could still have significant anti-malarial activity in vivo although they failed to in vitro. Most investigators usually progress to in vivo studies only when they observe significant antiplasmodial activity in vitro. This may explain the findings of a smaller number of in vivo studies in the current study. The investigation of the anti-malarial activities of plants should continue in vivo despite the dismal performance of the plants in vitro.
The current study revealed substantial inter-study variation in the antiplasmodial activity of several plant species. For example, considerable variation in the antiplasmodial activity was observed for Senna occidentalis, Adansonia digitata, Acanthospermum hispidum, Rotheca myricoides, Anogeissus leocarpus, Annona muricata, Ageratum conyzoides, Albizia coriaria, Ekebergia capensis, Flueggea virosa, Lippia javanica, Maytenus senegalensis, Morinda lucida, Picralima nitida, Trichilia emetica, Vernonia amydalina, and Vernonia colorata. The factors that could have accounted for these differences may include differences in the extraction solvent thus the extraction yield and extracted metabolite. With dichloromethane, mainly the apolar metabolites are extracted. In contrast, with methanol, from polar to moderate apolar metabolites are extracted.
Most (95.7%) of the studies used crude extract for their investigation and rarely the pure compounds (Additional file 1: Table S2 presents a summary of active compounds that have been identified from some of the plants). The finding of a majority of studies in Africa using only the crude extract of plants may be attributed to the absence of the necessary infrastructure to process the plant materials to get the pure compounds. Furthermore, there may be geographical differences in the areas where the plants were collected and this may also affect the activity of the same plant species. For example, despite using the same extraction solvent, the antiplasmodial activity of Acacia nilotica was moderate in South Africa and very good in Sudan. There was also variation between the different assay types. For example, the activities of Vernonia ambigua [211] and Annona senegalensis [204] have been reported to increase from inactive in vitro to very good in vivo. However, a few plant species including Alchornea cordifolia, and Zanthoxylum chalybeum, were observed to be consistently very good between studies. These plant species should be exploited further for their antiplasmodial activity. The activities of the plants were equally observed to increase with the isolation of the active compounds thus reinforcing the need for research into identifying the active compounds of African medicinal plants. The marked difference in the antiplasmodial activity of the crude extract of Artemisia annua and the pure compounds points out the issue that even the compounds which show only low potency and may be discarded from the initial screen for further development may still have active components with therapeutic potential [215]. The strain of the Plasmodium used may also be another factor accounting for the inter-study variation observed; studies using chloroquine-sensitive strains of the parasite like P. falciparum 3D7, D6, NF54 tend to report higher antiplasmodial activity compared to studies using chloroquine-resistant strains like P. falciparum W2, Dd5, K1 or D10.
This study revealed that only a few (26.3%) of the plants demonstrated some degree of toxicity. The families hosting the most toxic plant species were Lamiaceae, Anacardiaceae, Moraceae, and Meliaceae. The most toxic plants were Azadirachta indica and Vernonia amygdalina. The former [168] is one of the few plant species that demonstrated very good antiplasmodial activity in some studies. Other plants with high toxicity but very good antiplasmodial/anti-malarial activities include Arenga engleri [25], Celtis integrifolia [52], Ficus platyhylla [50], Gutenbergia cordifolia [21], Helchrysum cymosum [97], Microglossa pyrifolia [92], Opilia celtidifolia [52], Quassia Africana [103], Rumex abyssinicus [92], Clausena anisota [157], Icacina senegalensis [171], Abutilon grandiflorum [200], and Lannea schweinfurthii [205]. The isolation of the active compounds, which has to be done, could eliminate the toxicity, if not all, to a certain degree. For example, Salvia radula crude extract (of aerial parts) has been shown to demonstrate some degree of toxicity, but betulafolientriol oxide isolated from the plant was very active with little or no toxicity against human kidney epithelial cells [120]. There was also considerable variation in the toxicity between the assay types (in vitro or in vivo). As many as 32.8% of the plants demonstrated some level of toxicity in vitro meanwhile 26.7% were toxic in vivo. Since it is customary to evaluate toxicity at the in vitro level and toxic plants are discarded before in vivo evaluation, that may explain while fewer plants were toxic in vivo. Toxicity varied within the same plant species from study to study and could be attributed to differences in the study design as well as differences in the parts of the plants used for testing. From this study, the most toxicity was observed with the leaves. Also, a relationship could be established between toxicity and antiplasmodial activity; as the activity of the plant increases, the toxicity, on the other hand, was observed to decrease. Furthermore, albino mice and Vero E6 cells were the most commonly used assays in the evaluation of toxicity. Unfortunately, the authors could nt make a meaningful relationship between the type of assay and toxicity because of the fewer studies assessing the toxicity of the medicinal plants.
This study, however, is limited in that the analyses may have been compounded by the substantial inter-study variation in the methodologies used by different independent studies for the extraction of plant material, the overall extraction yield, the diversity of extracted metabolites as well as the geographical variations in the different sites used in the plant collection. However, the study has provided important baseline data that may be exploited by researchers in the field for the discovery and development of new anti-malarial drugs.
Conclusion
This study has revealed the slothful progress in the discovery and development of new anti-malarial drugs from African medicinal plants. Despite the encouraging activities demonstrated by the plants in vitro, fewer plants have been evaluated in vivo and just one clinical trial has been conducted so far with Cochlospermum planchonii (Bixaceae). The study also revealed considerable inter-study variation in the antiplasmodial activities of the plants, however, the activity of some plants including Alchornea cordifolia, Azadirachta indica, and Zanthoxylum chalybeum was consistently very good. The study demonstrates a relationship between antiplasmodial activity and toxicity whereby the toxicity of the plants decreases as the antiplasmodial activity increases. Besides, the active compounds were identified in just a handful of the plants. Therefore, there is a need for a policy-driven approach in the discovery and development of new anti-malarial drugs to subvert the rising resistance to the frontline anti-malarial drugs in the world.
Supplementary Information
Additional file 1: Table S1. In vitro and in vivo studies reporting inactive antiplasmodial or antimalarial activity. Table S2. List of active compounds identified from plants.
Acknowledgements
I would like to express my special appreciation and thanks to Professor Dr. Wanderley de Souza for his helpful comments.
Abbreviations
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- Nd
Not done
- Ns
Not specified
- SI
Selectivity Index
- LD50
Median lethal dose
- IC50
Half-maximal inhibitory concentration
- CC50
50% Cytotoxic concentration
- LC50
Lethal concentration
Authors’ contributions
All authors contributed equally to the study. All authors read and approved the manuscript.
Funding
N/A.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. World malaria report 2019. Geneva: World Health Organization; 2019. Accessed on 28/06/2021 at https://www.who.int/publications-detail/world-malaria-report-2019.
- 2.Kwenti ET. Malaria and HIV coinfection in sub-Saharan Africa: prevalence, impact, and treatment strategies. Res Rep Trop Med. 2018;9:123–136. doi: 10.2147/RRTM.S154501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwenti ET, Kukwah TA, Kwenti TDB, Nyassa BR, Dilonga MH, Enow-Orock G, et al. Comparative analysis of IgG and IgG subclasses against Plasmodium falciparum MSP-119 in children from five contrasting bioecological zones of Cameroon. Malar J. 2019;18:16. doi: 10.1186/s12936-019-2654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 6.Nagendrappa PB, Annamalai P, Naik M, Mahajan V, Mathur A, Susanta G, et al. A prospective comparative field study to evaluate the efficacy of a traditional plant-based malaria prophylaxis. J Intercult Ethnopharmacol. 2017;6:36–41. doi: 10.5455/jice.20161112021406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 9.Willcox ML. A clinical trial of 'AM', a Ugandan herbal remedy for malaria. J Public Health Med. 1999;21:318–324. doi: 10.1093/pubmed/21.3.318. [DOI] [PubMed] [Google Scholar]
- 10.Suswardany DL, Sibbritt DW, Supardi S, Pardosi JF, Chang S, Adams J. A cross-sectional analysis of traditional medicine use for malaria alongside free antimalarial drugs treatment amongst adults in high-risk malaria endemic provinces of Indonesia. PLoS One. 2017;12:e0173522. doi: 10.1371/journal.pone.0173522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahima HA, Imama IA, Bellob AM, Umara U, Muhammada S, Abdullahia SA. The potential of Nigerian medicinal plants as antimalarial agent: a review. Int J Sci Technol. 2012;2:600–605. [Google Scholar]
- 12.Zofou D, Kuete V, Titanji VPK. Antimalarial and other antiprotozoal products from African Medicinal plants. In: Medicinal plant research in Africa: pharmacology and chemistry. Kuete V, Ed. Chapt. 17. Amsterdam, Elsevier, 2013;661–709.
- 13.Lawal B, Shittu OK, Kabiru AY, Jigam AA, Umar MB, Berinyuy EB, et al. Potential antimalarials from African natural products: a review. J Intercult Ethnopharmacol. 2015;4:318–343. doi: 10.5455/jice.20150928102856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Wyk BE. A review of commercially important African medicinal plants. J Ethnopharmacol. 2015;176:118–134. doi: 10.1016/j.jep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Kaur R, Kaur H. Plant derived antimalarial agents. J Med Plants Studies. 2017;5:346–363. [Google Scholar]
- 16.Lemma MT, Ahmed AM, Elhady MT, Ngo HT, Vu TL, Sang TK, et al. Medicinal plants for in vitro antiplasmodial activities: a systematic review of literature. Parasitol Int. 2017;66:713–720. doi: 10.1016/j.parint.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deharo E, Bourdy G, Quenevo C, Munoz V, Ruiz G, Sauvain M. A search for natural bioactive compounds in Bolivia through multidisciplinary approach. Part V. Evaluation of the antimalarial activity of plants used by the Tacana Indians. J Ethnopharmacol. 2001;77:91–8. doi: 10.1016/S0378-8741(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 19.Becker JVW, van der Merwe MM, van Brummelen AC, Pillay P, Crampton BG, Mmutlane EM, et al. In vitro anti-plasmodial activity of Dicoma anomala subsp gerrardii (Asteraceae): identification of its main active constituent, structure-activity relationship studies, and gene expression profiling. Malar J. 2011;10:295. doi: 10.1186/1475-2875-10-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gessler MC, Nkunya MH, Mwasumbi LB, Heinrich M, Tanner M. Screening Tanzanian medicinal plants for antimalarial activity. Acta Trop. 1994;56:65–77. doi: 10.1016/0001-706X(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 21.Koch A, Tamez P, Pezzuto J, Soejarto D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J Ethnopharmacol. 2005;101:95–99. doi: 10.1016/j.jep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Clarkson C, Maharaj VJ, Crouch NR, Grace OM, Pillay P, Matsabisa MG, et al. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J Ethnopharmacol. 2004;92:177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 23.El Tahir A, Satti GM, Khalid SA. Antiplasmodial activity of selected Sudanese medicinal plants with emphasis on Maytenus senegalensis (Lam.) Exell. J Ethnopharmacol. 1999;64:227–33. doi: 10.1016/S0378-8741(98)00129-9. [DOI] [PubMed] [Google Scholar]
- 24.Muthaura CN, Keriko JM, Mutai C, Yenesew A, Gathirwa JW, Irungu BN, et al. Antiplasmodial potential of traditional phytotherapy of some remedies used in treatment of malaria in Meru-Tharaka Nithi County of Kenya. J Ethnopharmacol. 2015;175:315–323. doi: 10.1016/j.jep.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Prozesky EA, Meyer JJ, Louw AI. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected South African plants. J Ethnopharmacol. 2001;76:239–245. doi: 10.1016/S0378-8741(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 26.Muthaura CN, Keriko JM, Mutai C, Yenesew A, Gathirwa JW, Irungu BN, et al. Antiplasmodial potential of traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan Coast. J Ethnopharmacol. 2015;170:148–157. doi: 10.1016/j.jep.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Sanon S, Ollivier E, Azas N, Mahiou V, Gasquet M, Ouattara CT, et al. Ethnobotanical survey and in vitro antiplasmodial activity of plants used in traditional medicine in Burkina Faso. J Ethnopharmacol. 2003;86:143–147. doi: 10.1016/S0378-8741(02)00381-1. [DOI] [PubMed] [Google Scholar]
- 28.Zirihi-Guédé N, Mambu L, Guédé-Guina F, Bodo B, Grellier P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J Ethnopharmacol. 2005;98:281–285. doi: 10.1016/j.jep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Koukouikila-Koussounda F, Abena AA, Nzoungani A, Mombouli JV, Ouamba JM, Kun J, et al. In vitro evaluation of antiplasmodial activity of extracts of Acanthospermum hispidum DC (Asteraceae) and Ficus thonningii Blume (Moraceae), two plants used in traditional medicine in the Republic of Congo. Afr J Tradit Complement Altern Med. 2012;10:270–276. [PMC free article] [PubMed] [Google Scholar]
- 30.Owuor BO, Ochanda JO, Kokwaro JO, Cheruiyot AC, Yeda RA, Okudo CA, et al. In vitro antiplasmodial activity of selected Luo and Kuria medicinal plants. J Ethnopharmacol. 2012;144:779–781. doi: 10.1016/j.jep.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 31.Malebo HM, Tanja W, Cal M, Swaleh SAM, Omolo MO, Hassanali A, et al. Antiplasmodial, anti-trypanosomal, anti-leishmanial and cytotoxicity activity of selected Tanzanian medicinal plants. Tanzan J Health Res. 2009;11:226–234. doi: 10.4314/thrb.v11i4.50194. [DOI] [PubMed] [Google Scholar]
- 32.Annan K, Sarpong K, Asare C, Dickson R, Amponsah KI, Gyan B, et al. In vitro anti-plasmodial activity of three herbal remedies for malaria in Ghana: Adenia cissampeloides (Planch.) Harms., Termina liaivorensis A. Chev, and Elaeis guineensis Jacq. Pharmacognosy Res. 2012;4:225–9. doi: 10.4103/0974-8490.102270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lekana-Douki JB, Liabagui SLO, Bongui JB, Zatra R, Lebibi J, Toure-Ndouo FS. In vitro antiplasmodial activity of crude extracts of Tetrapleura tetraptera and Copaifera religiosa. BMC Res Notes. 2011;4:506. doi: 10.1186/1756-0500-4-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed EHM, Nour BYM, Mohammed YG, Khalid HS. Antiplasmodial activity of some medicinal plants used in Sudanese fFolk-medicine. Environ Health Insights. 2010;4:1–6. doi: 10.4137/EHI.S4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuria KA, Chepkwony H, Govaerts C, Roets E, Busson R, De Witte P, et al. The antiplasmodial activity of isolates from Ajuga remota. J Nat Prod. 2002;65:789–793. doi: 10.1021/np0104626. [DOI] [PubMed] [Google Scholar]
- 36.Lasisi AA, Olayiwola MA, Balogun SA, Akinloye OA, Ojo DA. Phytochemical composition, cytotoxicity and in vitro antiplasmodial activity of fractions from Alafia barteri olive (Hook F. Icon)-Apocynaceae. J Saudi Chem Soc. 2016;20:2–6. doi: 10.1016/j.jscs.2012.05.003. [DOI] [Google Scholar]
- 37.Bapela MJ, Meyer JJ, Kaiser M. In vitro antiplasmodial screening of ethnopharmacologically selected South African plant species used for the treatment of malaria. J Ethnopharmacol. 2014;156:370–373. doi: 10.1016/j.jep.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Banzouzi JT, Prado R, Menan H, Valentin A, Roumestan C, Mallie M, et al. In vitro antiplasmodial activity of extracts of Alchornea cordifolia and identification of an active constituent: ellagic acid. J Ethnopharmacol. 2002;81:399–401. doi: 10.1016/S0378-8741(02)00121-6. [DOI] [PubMed] [Google Scholar]
- 39.Mustofa, Valentin A, Benoit-Vical F, Pélissier Y, Koné-Bamba D, Mallié M. Antiplasmodial activity of plant extracts used in West African traditional medicine. J Ethnopharmacol. 2000;73:145–51. [DOI] [PubMed]
- 40.Musuyu Muganza D, Fruth BI, Nzunzu Lami J, Mesia GK, Kambu OK, Tona GL, et al. In vitro antiprotozoal and cytotoxic activity of 33 ethonopharmacologically selected medicinal plants from Democratic Republic of Congo. J Ethnopharmacol. 2012;141:301–308. doi: 10.1016/j.jep.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 41.Abdissa D, Geleta G, Bacha K, Abdissa N. Phytochemical investigation of Aloe pulcherrima roots and evaluation for its antibacterial and antiplasmodial activities. PLoS ONE. 2017;12:e0173882. doi: 10.1371/journal.pone.0173882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iyiola OA, Tijani AY, Lateef KM. Antimalarial activity of ethanolic stem barks extract of Alstonia boonei in mice. Asian J Biol Sci. 2011;4:235–243. doi: 10.3923/ajbs.2011.235.243. [DOI] [Google Scholar]
- 43.Zirihi-Guédé N, N’guessan K, Etien Dibié T, Grellier P. Ethnopharmacological study of plants used to treat malaria, in traditional medicine, by Bete populations of Issia (Côte d’Ivoire) J Pharm Sci Res. 2010;2:216–27. [Google Scholar]
- 44.Lumpu SL, Kikueta CM, Tshodi ME, Mbenza AP, Kambu OK, Mbamu BM, et al. Antiprotozoal screening and cytotoxicity of extracts and fractions from the leaves, stem barks and root barks of Alstonia congensis. J Ethnopharmacol. 2013;148:724–727. doi: 10.1016/j.jep.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Hout S, Chea A, Bun SS, Elias R, Gasquet M, Timon-David P, et al. Screening of selected indigenous plants of Cambodia for antiplasmodial activity. J Ethnopharmacol. 2006;107:12–18. doi: 10.1016/j.jep.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 46.Lusakibanza M, Mesia G, Tona G, Karemere S, Lukuka A, Tits M, et al. In vitro and in vivo antimalarial and cytotoxic activity of five plants used in Congolese traditional medicine. J Ethnopharmacol. 2010;129:398–402. doi: 10.1016/j.jep.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Yamthe LRT, Fokou PVT, Mbouna CDJ, Keumoe R, Ndjakou BL, Djouonzo PT, et al. Extracts from Annona muricata L. and Annona reticulata L. (Annonaceae) potently and selectively inhibit Plasmodium falciparum. Medicines (Basel). 2015;2:55–66. doi: 10.3390/medicines2020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ménan H, Banzouzi J-T, Hocquette A, Pélissier Y, Blache Y, Koné M, et al. Antiplasmodial activity and cytotoxicity of plants used in West African traditional medicine for the treatment of malaria. J Ethnopharmacol. 2006;105:131–136. doi: 10.1016/j.jep.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 49.Boyom FF, Fokou PV, Yamthe LR, Mfopa AN, Kemgne EM, Mbacham WF, et al. Potent antiplasmodial extracts from Cameroonian Annonaceae. J Ethnopharmacol. 2011;134:717–724. doi: 10.1016/j.jep.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Shuaibu MN, Wuyep PA, Yanagi T, Hirayama K, Tanaka T, Kouno I. The use of microfluorometric method for activity-guided isolation of antiplasmodial compound from plant extracts. Parasitol Res. 2008;102:1119–1127. doi: 10.1007/s00436-008-0879-6. [DOI] [PubMed] [Google Scholar]
- 51.Vonthron-Sénécheau C, Weniger B, Ouattara M, Bi FT, Kamenan A, Lobstein A, et al. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected Ivorian plants. J Ethnopharmacol. 2003;87:221–225. doi: 10.1016/S0378-8741(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 52.Sanon S, Gansane A, Ouattara LP, Traore A, OueDRaogo IN, Tiono A, et al. In vitro antiplasmodial and cytotoxic properties of some medicinal plants from western Burkina Faso. Afr J Lab Med. 2013;2:81. doi: 10.4102/ajlm.v2i1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chukwujekwu JC, van Staden J, Smith P, Meyer JJM. Antibacterial, anti-inflammatory, and antimalarial activities of some Nigerian medicinal plants. S Afr J Bot. 2005;71:316–325. doi: 10.1016/S0254-6299(15)30105-8. [DOI] [Google Scholar]
- 54.Kraft C, Jenett-Siems K, Siems K, Jakupovic J, Mavi S, Bienzle U, et al. In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytother Res. 2003;17:123–128. doi: 10.1002/ptr.1066. [DOI] [PubMed] [Google Scholar]
- 55.Boyom FF, Kemgne EM, Tepongning R, Ngouana V, Mbacham WF, Tsamo E, et al. Antiplasmodial activity of extracts from seven medicinal plants used in malaria treatment in Cameroon. J Ethnopharmacol. 2009;123:483–488. doi: 10.1016/j.jep.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Waako PJ, Katuura E, Smith P, Folb P. East African medicinal plants as a source of lead compounds for the development of new antimalarial drugs. Afr J Ecol. 2007;45:102–106. doi: 10.1111/j.1365-2028.2007.00752.x. [DOI] [Google Scholar]
- 57.Benoit F, Valentin A, Pelissier Y, Diafouka F, Marion C, Kone-Bamba D, et al. In vitro antimalarial activity of vegetal extracts used in West African traditional medicine. Am J Trop Med Hyg. 1996;54:67–71. doi: 10.4269/ajtmh.1996.54.67. [DOI] [PubMed] [Google Scholar]
- 58.El-Tahir A, Satti GM, Khalid SA. Antiplasmodial activity of selected Sudanese medicinal plants with emphasis on Acacia nilotica. Phytother Res. 1999;13:474–478. doi: 10.1002/(SICI)1099-1573(199909)13:6<474::AID-PTR482>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 59.MacKinnon S1, Durst T, Arnason JT, Angerhofer C, Pezzuto J, SanchezVindas PE, et al. Antimalarial activity of tropical Meliaceae extracts and Gedunin derivatives. J Nat Prod. 1997;60:336–41. [DOI] [PubMed]
- 60.Connelly MP, Fabiano E, Patel IH, Kinyanjui SM, Mberu EK, Watkins WM. Antimalarial activity in crude extracts of Malawian medicinal plants. Ann Trop Med Parasitol. 1996;90:597–602. doi: 10.1080/00034983.1996.11813089. [DOI] [PubMed] [Google Scholar]
- 61.Ngwira KJ, Maharaj VJ, Mgani QA. In vitro antiplasmodial and HIV-1 neutralization activities of root and leaf extracts from Berberis holstii. J Herb Med. 2015;5:30–35. doi: 10.1016/j.hermed.2014.12.001. [DOI] [Google Scholar]
- 62.Jansen O, Angenot L, Tits M, Nicolas JP, De Mol P, Nikiéma JB, et al. Evaluation of 13 selected medicinal plants from Burkina Faso for their antiplasmodial properties. J Ethnopharmacol. 2010;130:143–150. doi: 10.1016/j.jep.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 63.Benoit-Vical F, Soh PN, Saléry M, Harguem L, Poupat C, Nongonierma R. Evaluation of Senegalese plants used in malaria treatment: focus on Chrozophora senegalensis. J Ethnopharmacol. 2008;116:43–48. doi: 10.1016/j.jep.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 64.Ogunlanaa OO, Kimb H-S, Watayab Y, Olagunjuc JO, Akindahunsid AA, Tan NH. Antiplasmodial flavonoid from young twigs and leaves of Caesalpinia bonduc (Linn) Roxb. J Chem Pharm Res. 2015;7:931–937. [Google Scholar]
- 65.Weniger B, Lagnika L, Vonthron-Sénécheau C, Adjobimey T, Gbenou J, Moudachirou M, et al. Evaluation of ethnobotanically selected Benin medicinal plants for their in vitro antiplasmodial activity. J Ethnopharmacol. 2004;90:279–284. doi: 10.1016/j.jep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Melariri P, Campbell W, Etusim P, Smith P. Antiplasmodial properties and bioassay-guided fractionation of ethyl acetate extracts from Carica papaya leaves. J Parasitol Res. 2011;2011:104954. doi: 10.1155/2011/104954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kayembe JS, Taba KM, Ntumba K, Tshiongo MTC, Kazadi TK. In vitro antimalarial activity of 20 quinones isolated from four plants used by traditional healers in the Democratic Republic of Congo. J Med Plant Res. 2010;4:991–994. [Google Scholar]
- 68.Ramalhete C, Lopes D, Mulhovo S, Rosário VE, Ferreira MJU. Antimalarial activity of some plants traditionally used in Mozambique. Workshop Plantas Medicinais e Fitoterapêuticas nos Trópicos.IICT /CCCM, 29, 30 e 31 de Outubro de 2008.
- 69.Tona L, Cimanga RK, Mesia K, Musuamba CT, De Bruyne T, Apers S, et al. In vitro antiplasmodial activity of extracts and fractions from seven medicinal plants used in the Democratic Republic of Congo. J Ethnopharmacol. 2004;93:27–32. doi: 10.1016/j.jep.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 70.Gbeassor M, Kossou Y, Amegbo K, de Souza C, Koumaglo K, Denke A. Antimalarial effects of eight African medicinal plants. J Ethnopharmacol. 1989;25:115–118. doi: 10.1016/0378-8741(89)90051-2. [DOI] [PubMed] [Google Scholar]
- 71.Afoulous S, Ferhout H, Raoelison EG, Valentin A, Moukarzel B, Couderc F, et al. Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaf essential oil of Cedrelopsis grevei. Food Chem Toxicol. 2013;56:352–362. doi: 10.1016/j.fct.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Irungu BN, Rukunga GM, Mungai GM, Muthaura CN. In vitro antiplasmodial and cytotoxicity activities of 14 medicinal plants from Kenya. S Afr J Bot. 2007;73:204–207. doi: 10.1016/j.sajb.2006.11.004. [DOI] [Google Scholar]
- 73.do Céu de Madureira M, Paula Martins A, Gomes M, Paiva J, Proença da Cunha A, do Rosário V. Antimalarial activity of medicinal plants used in traditional medicine in S. Tomé and Príncipe islands. J Ethnopharmacol. 2002;81:23–9. [DOI] [PubMed]
- 74.Rukunga GM, Gathirwa JW, Omar SA, Muregi FW, Muthaura CN, Kirira PG, et al. Anti-plasmodial activity of the extracts of some Kenyan medicinal plants. J Ethnopharmacol. 2009;121:282–285. doi: 10.1016/j.jep.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 75.Lacroix D, Prado S, Kamoga D, Kasenene J, Namukobe J, Krief S, et al. Antiplasmodial and cytotoxic activities of medicinal plants traditionally used in the village of Kiohima Uganda. J Ethnopharmacol. 2011;133:850–855. doi: 10.1016/j.jep.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 76.Muregi FW, Chhabra SC, Njagi EN, Lang'at-Thoruwa CC, Njue WM, Orago AS, et al. Anti-plasmodial activity of some Kenyan medicinal plant extracts singly and in combination with chloroquine. Phytother Res. 2004;18:379–384. doi: 10.1002/ptr.1439. [DOI] [PubMed] [Google Scholar]
- 77.Adia MM, Emami SN, Byamukama R, Faye I, Borg-Karlson AK. Antiplasmodial activity and phytochemical analysis of extracts from selected Ugandan medicinal plants. J Ethnopharmacol. 2016;186:14–19. doi: 10.1016/j.jep.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 78.Lamien-Meda A, Kiendrebeogo M, Compaoré M, Meda RN, Bacher M, Koenig K, et al. Quality assessment and antiplasmodial activity of West African Cochlospermum species. Phytochemistry. 2015;119:51–61. doi: 10.1016/j.phytochem.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Benoit F, Valentin A, Pélissier Y, Marion C, Dakuyo Z, Mallié M, et al. Antimalarial activity in vitro of Cochlospermum tinctorium tubercle extracts. Trans R Soc Trop Med Hyg. 1995;89:217–218. doi: 10.1016/0035-9203(95)90502-2. [DOI] [PubMed] [Google Scholar]
- 80.Zofou D, Kengne ABO, Tene M. Ngemenya MN, Tane P, Titanji VP. In vitro antiplasmodial activity and cytotoxicity of crude extracts and compounds from the stem barks of Kigelia africana (Lam.) Benth (Bignoniaceae). Parasitol Res. 2011;108:1383–90. [DOI] [PubMed]
- 81.Paulo A, Gomes ET, Houghton PJ. New alkaloids from Cryptolepis sanguinolenta. J Nat Prod. 1995;58:1485–1491. doi: 10.1021/np50124a002. [DOI] [Google Scholar]
- 82.Kirby GC, Paine A, Warhurst DC, Noamese BK, Phillipson JD. In vitro and in vivo antimalarial activity of cryptolepine, a plant-derived indoloquinoline. Phytother Res. 1995;9:359–363. doi: 10.1002/ptr.2650090510. [DOI] [Google Scholar]
- 83.Cimanga K, De Bruyne T, Pieters L, Vlietinck AJ, Turger CA. In vitro and in vivo antiplasmodial activity of cryptolepine and related alkaloids from Cryptolepis sanguinea. J Nat Prod. 1997;60:688–691. doi: 10.1021/np9605246. [DOI] [PubMed] [Google Scholar]
- 84.Grellier P, Ramiaramanana L, Millerioux V, Deharo E, Schrével J, Frappier F, et al. Antimalarial activity of cryptolepine and isocryptolepine, alkaloids isolated from Cryptolepis sanguinolenta. Phytother Res. 1996;10:317–321. doi: 10.1002/(SICI)1099-1573(199606)10:4<317::AID-PTR858>3.0.CO;2-0. [DOI] [Google Scholar]
- 85.Zofou D, Tematio EL, Ntie-Kang F, Tene M, Ngemenya MN, Tane P, et al. New antimalarial hits from Dacryodes edulis (Burseraceae) - Part I: Isolation, in vitro activity, in silico “drug-likeness” and pharmacokinetic profiles. PLoS ONE. 2013;8:e79544. doi: 10.1371/journal.pone.0079544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nafuka SN, Mumbengegwi DR. Phytochemical analysis and in vitro anti-plasmodial activity of selected ethnomedicinal plants used to treat malaria associated symptoms in Northern Namibia. Int Sci Technol J Namibia. 2013;2:78–93. [Google Scholar]
- 87.Jansen O, Tits M, Nicolas ALJP, De Mol P, Nikiema J-B, Frédérich M. Anti-plasmodial activity of Dicoma tomentosa (Asteraceae) and identification of urospermal A-15-O-acetate as the main active compound. Malar J. 2012;11:289. doi: 10.1186/1475-2875-11-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olasehinde GI, Ojurongbe O, Adeyeba AO, Fagade OE, Valecha N, Ayanda IO, et al. In vitro studies on the sensitivity pattern of Plasmodium falciparum to antimalarial drugs and local herbal extracts. Malar J. 2014;13:63. doi: 10.1186/1475-2875-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bickii J, Tchouya GRF, Tchouankeu JC, Tsamo E. Antimalarial activity in crude extracts of some Cameroonian medicinal plants. Afr J Tradit Complement Altern Med. 2007;4:107–111. doi: 10.4314/ajtcam.v4i1.31200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, Murakami N, Ji H, Abreu P, Zhang S. Antimalarial flavonol glycosides from Euphorbiahirta. Pharm Biol. 2007;45:278–281. doi: 10.1080/13880200701214748. [DOI] [Google Scholar]
- 91.Kaou AM, Mahiou-Leddet V, Hutter S, Aïnouddine S, Hassani S, Yahaya I, et al. Antimalarial activity of crude extracts from nine African medicinal plants. J Ethnopharmacol. 2008;116:74–83. doi: 10.1016/j.jep.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 92.Muganga R, Angenot L, Tits M, Frédérich M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J Ethnopharmacol. 2010;128:52–57. doi: 10.1016/j.jep.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 93.Traore-Keita F, Gasquet M, Di Giorgio C, Ollivier E, Delmas F, Keita A, et al. Antimalarial activity of four plants used in traditional medicine in Mali. Phytother Res. 2000;14:45–47. doi: 10.1002/(SICI)1099-1573(200002)14:1<45::AID-PTR544>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 94.Ancolio C, Azas N, Mahiou V, Ollivier E, Di Giorgio C, Keita A, et al. Antimalarial activity of extracts and alkaloids isolated from six plants used in traditional medicine in Mali and Sao Tome. Phytother Res. 2002;16:646–649. doi: 10.1002/ptr.1025. [DOI] [PubMed] [Google Scholar]
- 95.Nyambati GK, Lagat ZO, Maranga RO, Samuel M, Ozwara H. In vitro anti-plasmodial activity of Rubia cordifolia, Harrizonia abyssinica, Leucas calostachys Olive and Sanchus schweinfurthii medicinal plants. J Appl Pharm Sci. 2013;3:57–62. [Google Scholar]
- 96.Afoulous S, Ferhout H, Raoelison EG, Valentin A, Moukarzel B, Couderc F, et al. Helichrysum gymnocephalum essential oil: chemical composition and cytotoxic, antimalarial and antioxidant activities, attribution of the activity origin by correlations. Molecules. 2011;16:8273–8291. doi: 10.3390/molecules16108273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Vuuren SF, Viljoen AM, Van Zyl RL, Van Heerden FR, Baser KHC. The antimicrobial, antimalarial and toxicity profiles of helihumulone, Leaf essential oil and extracts of Helichrysum cymosum (L.) D. Don subsp. cymosum. S Afr J Bot. 2006;72:287–290. doi: 10.1016/j.sajb.2005.07.007. [DOI] [Google Scholar]
- 98.Boyom FF. Composition and anti-plasmodial activities of essential oils from some Cameroonian medicinal plants. Phytochemistry. 2003;64:1269–1275. doi: 10.1016/j.phytochem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Fotie J, Bohle DS, Leimanis ML, Georges E, Rukunga G, Nkengfack AE. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda. J Nat Prod. 2006;69:62–67. doi: 10.1021/np050315y. [DOI] [PubMed] [Google Scholar]
- 100.Sarr SO, Perrotey S, Fall I, Ennahar S, Zhao M, Diop YM, et al. Icacina senegalensis (Icacinaceae), traditionally used for the treatment of malaria, inhibits in vitro Plasmodium falciparum growth without host cell toxicity. Malar J. 2011;10:85. doi: 10.1186/1475-2875-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bickii J, Njifutie N, Foyere JA, Basco LK, Ringwald P. In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae) J Ethnopharmacol. 2000;69:27–33. doi: 10.1016/S0378-8741(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 102.Wube AA, Bucar F, Asres K, Gibbons S, Rattray L, Croft SL. Antimalarial compounds from Kniphofia foliosa roots. Phytother Res. 2005;19:472–476. doi: 10.1002/ptr.1635. [DOI] [PubMed] [Google Scholar]
- 103.Mbatchi SF, Mbatchi B, Banzouzi JT, Bansimba T, Nsonde Ntandou GF, Ouamba JM, et al. In vitro antiplasmodial activity of 18 plants used in Congo Brazzaville traditional medicine. J Ethnopharmacol. 2006;104:168–174. doi: 10.1016/j.jep.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 104.Oketch-Rabah HA, Dossaji SF, Mberu EK. Antimalarial activity of some Kenyan medicinal plants. Pharm Biol. 1999;37:329–334. doi: 10.1076/phbi.37.5.329.6053. [DOI] [Google Scholar]
- 105.Ramalhete C, da Cruz FP, Mulhovo S, Sousa IJ, Fernandes MX, Prudêncio M, et al. Dual-stage triterpenoids from an African medicinal plant targeting the malaria parasite. Bioorg Med Chem. 2014;22:3887–3890. doi: 10.1016/j.bmc.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 106.BenoitVical F, Valentin A, Cournac V, Pélissier Y, Mallié M, Bastide JM. In vitro antiplasmodial activity of stem and root extracts of Nauclea latifolia S.M. (Rubiaceae) J Ethnopharmacol. 1998;61:173–8. doi: 10.1016/S0378-8741(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 107.Mesia K, Cimanga RK, Dhooghe L, Cos P, Apers S, Totté J, et al. Antimalarial activity and toxicity evaluation of a quantified Nauclea pobeguinii extract. J Ethnopharmacol. 2010;131:6. doi: 10.1016/j.jep.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Gbeassor M, Kedjagni AY, Koumaglo K, de Soma C, Agbo K, Aklikokou K, et al. In vitro antimalarial activity of six medicinal plants. Phytother Res. 1990;4:115–117. doi: 10.1002/ptr.2650040309. [DOI] [Google Scholar]
- 109.Karim T, Béourou S, Touré AO, Ouattara K, Meité S, Ako A, et al. Antioxidant activities and estimation of the phenols and flavonoids content in the extracts of medicinal plants used to treat malaria in Ivory Coast. Int J Curr Microbiol App Sci. 2015;4:862–874. [Google Scholar]
- 110.Koudouvo K, Karou SD, Ilboudo DP, Kokou K, Essien K, Aklikokou K, et al. In vitro antiplasmodial activity of crude extracts from Togolese medicinal plants. Asian Pac J Trop Med. 2011;4:129–132. doi: 10.1016/S1995-7645(11)60052-7. [DOI] [PubMed] [Google Scholar]
- 111.Appiah-Opong R, Nyarko AK, Dodoo D, Gyang FN, Koram KA, Ayisi NK. Antiplasmodial activity of extracts of Tridax procumbens and Phyllanthus amarus in in vitro Plasmodium falciparum culture systems. Ghana Med J. 2011;45:143–150. [PMC free article] [PubMed] [Google Scholar]
- 112.Komlaga G, Cojean S, Dickson RA, Mehdi A, Beniddir MA, SuyyaghAlbouz S, et al. Antiplasmodial activity of selected medicinal plants used to treat malaria in Ghana. Parasitol Res. 2016;115:3185–95. doi: 10.1007/s00436-016-5080-8. [DOI] [PubMed] [Google Scholar]
- 113.Falodun A, Imieje V, Erharuyi O, Ahomafor J, Jacob MR, Khan SI, et al. Evaluation of three medicinal plant extracts against Plasmodium falciparum and selected microganisms. Afr J Tradit Complement Altern Med. 2014;11:142–146. doi: 10.4314/ajtcam.v11i4.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.François G, Aké Assi L, Holenz J, Bringmann G. Constituents of Picralima nitida display pronounced inhibitory activities against asexual erythrocytic forms of Plasmodium falciparum in vitro. J Ethnopharmacol. 1996;54:113–117. doi: 10.1016/S0378-8741(96)01456-0. [DOI] [PubMed] [Google Scholar]
- 115.Gbedema SY, Bayor MT, Annan K, Wright CW. Clerodane diterpenes from Polyalthia longifolia (sonn.) Thw. Var. Pendula: potential antimalarial agents for drug resistant Plasmodium falciparum infection. J Ethnopharmacol. 2015;169:176–82. doi: 10.1016/j.jep.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 116.Annan K, Ekuadzi E, Asare C, Sarpong K, Pistorius D, Oberer L, et al. Antiplasmodial constituents from the stem barks of Polyalthia longifolia var pendula. Phytochem Lett. 2015;11:28–31. doi: 10.1016/j.phytol.2014.10.028. [DOI] [Google Scholar]
- 117.Orwa JA, Ngeny L, Mwikwabe NM, Ondicho J, Jondiko IJ. Antimalarial and safety evaluation of extracts from Toddalia asiatica (L) Lam. (Rutaceae) J Ethnopharmacol. 2013;145:587–90. doi: 10.1016/j.jep.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 118.Karou D, Dicko MH, Sanon S, Simpore J, Traore AS. Antimalarial activity of Sida acuta Burm. F. (Malvaceae) and Pterocarpus erinaceus Poir. (Fabaceae) J Ethnopharmacol. 2003;89:291–4. doi: 10.1016/j.jep.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 119.Muregi FW, Chhabra SC, Njagi EN, Lang'at-Thoruwa CC, Njue WM, Orago AS, et al. In vitro antiplasmodial activity of some plants used in Kisii, Kenya against malaria and their chloroquine potentiation effects. J Ethnopharmacol. 2003;84:235–239. doi: 10.1016/S0378-8741(02)00327-6. [DOI] [PubMed] [Google Scholar]
- 120.Kamatou GPP, Van Zyl RL, Davids H, Van Heerden FR, Lourens ACU, Viljoen AM. Antimalarial and anticancer activities of selected South African Salvia species and isolated compounds from S. radula. S Afr J Bot. 2008;74:238–43. doi: 10.1016/j.sajb.2007.08.001. [DOI] [Google Scholar]
- 121.Bah S, Jäger AK, Adsersen A, Diallo D, Paulsen BS. Antiplasmodial and GABA(A)-benzodiazepine receptor binding activities of five plants used in traditional medicine in Mali. West Africa J Ethnopharmacol. 2007;110:451–457. doi: 10.1016/j.jep.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 122.Carraz M, Jossang A, Franetich J-F, Siau A, Ciceron L, Hannoun L, et al. A plant-derived morphinan as a novel lead compound active against malaria liver stages. PLoS Med. 2006;3:e513. doi: 10.1371/journal.pmed.0030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Niass O, Sarr SO, Dieye B, Diop A, Diop YM. In vitro assessment of the antiplasmodial activity of three extracts used in local traditional medicine in Saloum (Senegal) Eur Sci J. 2016;12:157–165. [Google Scholar]
- 124.Kigondu EV, Rukunga GM, Keriko JM, Tonui WK, Gathirwa JW, Kirira PG, et al. Anti-parasitic activity and cytotoxicity of selected medicinal plants from Kenya. J Ethnopharmacol. 2009;123:504–509. doi: 10.1016/j.jep.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 125.Gakunju DM, Mberu EK, Dossaji SF, Gray AI, Waigh RD, Waterman PG, et al. Potent antimalarial activity of the alkaloid nitidine, isolated from a Kenyan herbal remedy. Antimicrob Agents Chemother. 1995;39:2606–2609. doi: 10.1128/AAC.39.12.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Omoregie ES, Pal A, Sisodia B. In vitro antimalarial and cytotoxic activities of leaf extracts of Vernonia amygdalina (Del.) Niger J Basic Appl Sci. 2011;19:121–6. [Google Scholar]
- 127.Shaa KK, Oguche S, Watila IM, Ikpa TF. In vitro antimalarial activity of the extracts of Vernonia amygdalina commonly used in traditional medicine in Nigeria. Sci World J. 2011;6:5–9. [Google Scholar]
- 128.Toyang NJ, Krause MA, Fairhurst RM, Tane P, Bryant J, Verpoorte R. Antiplasmodial activity of sesquiterpene lactones and a sucrose ester from Vernonia guineensis Benth (Asteraceae) J Ethnopharmacol. 2013;147:618–21. doi: 10.1016/j.jep.2013.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goodman CD, Austarheim I, Mollard V, Mikolo B, Malterud KE, McFadden GI, et al. Natural products from Zanthoxylum heitzii with potent activity against the malaria parasite. Malar J. 2016;15:481. doi: 10.1186/s12936-016-1533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Randrianarivelojosia M, Rasidimanana VT, Rabarison H, Cheplogoi PK, Ratsimbason M, Mulholland DA, et al. Plants traditionally prescribed to treat tazo (malaria) in the eastern region of Madagascar. Malar J. 2003;2:25. doi: 10.1186/1475-2875-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okokon JE, Augustine NB, Mohanakrishnan D. Antimalarial, antiplasmodial and analgesic activities of root extract of Alchornea laxiflora. Pharm Biol. 2017;55:1022–1031. doi: 10.1080/13880209.2017.1285947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alli LA, Adesokan AA, Salawu OA, Akanji MA, Tijani AY. Anti-plasmodial activity of aqueous root extract of Acacia nilotica. Afr J Biochem Res. 2011;5:214–219. [Google Scholar]
- 133.Jigam AA, Akanya HO, Dauda BEN, Okogun JO. Polygalloyltannin isolated from the roots of Acacia nilotica Del. (Leguminoseae) is effective against Plasmodium berghei in mice. J Med Plant Res. 2010;4:1169–75. [Google Scholar]
- 134.Adeoye AO, Bewaji CO. Chemopreventive, and remediation effect of Adansonia digitata L Baobab (Bombacaceae) stem barks extracts in mouse model malaria. J Ethnopharmacol. 2018;210:31–8. doi: 10.1016/j.jep.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 135.Musila MF, Dossaji SF, Nguta JM, Lukhoba CW, Munyao JM. In vivo antimalarial activity, toxicity and phytochemical screening of selected antimalarial plants. J Ethnopharmacol. 2013;146:557–561. doi: 10.1016/j.jep.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 136.Ukwe VC, Epueke EA, Ekwunife OI, Okoye TC, Akudor GC, Ubaka CM. Antimalarial activity of aqueous extract and fractions of leaves of Ageratum conyzoides in mice infected with Plasmodium berghei. Int J Pharm Sci. 2010;2:33–38. [Google Scholar]
- 137.Rukunga GM, Muregi FW, Tolo FM, Omar SA, Mwitari P, Muthaura CN, et al. The antiplasmodial activity of spermine alkaloids isolated from Albizia gummifera. Fitoterapia. 2007;78:455–459. doi: 10.1016/j.fitote.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 138.Oladosu IA, Balogun SO, Ademowo GO. Phytochemical screening, antimalarial and histopathological studies of Allophylus africanus and Tragia benthamii. Chin J Nat Med. 2013;11:371–376. doi: 10.3724/SP.J.1009.2013.00371. [DOI] [PubMed] [Google Scholar]
- 139.Teka T, Bisrat D, Yeshak MY, Asres K. Antimalarial activity of the chemical constituents of the leaves latex of Aloe pulcherrima Gilbert and Sebsebe. Molecules. 2016;21:1415. doi: 10.3390/molecules21111415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Akpan EJ, Okokon JE, Etuk IC. Antiplasmodial and antipyretic studies on root extracts of Anthocleista djalonensis against Plasmodium berghei. Asian Pac J Trop Dis. 2012;2:36–42. doi: 10.1016/S2222-1808(12)60009-7. [DOI] [Google Scholar]
- 141.Ene AC, Ameh DA, Kwanashie HO, Agomo PU, Atawodi SE. Preliminary in vivo antimalarial screening of petroleum ether, chloroform, and methanol extracts of fifteen plants grown in Nigeria. J Pharmacol Toxicol. 2008;3:254–260. doi: 10.3923/jpt.2008.254.260. [DOI] [Google Scholar]
- 142.Christian AG, Mfon AG, Dick EA, David-Oku E, Akpan JL, Chukwuma EB. Antimalarial potency of the leaf extract of Aspilia africana (Pers.) C.D. Adams. Asian Pac J Trop Med. 2012;2:126–9. doi: 10.1016/S1995-7645(12)60010-8. [DOI] [PubMed] [Google Scholar]
- 143.Gathirwa JW, Rukunga GM, Mwitari PG, Mwikwabe NM, Kimani CW, Muthaura CN, et al. Traditional herbal antimalarial therapy in Kilifi district. Kenya J Ethnopharmacol. 2011;134:434–442. doi: 10.1016/j.jep.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 144.Tepongning RN, Mbah JN, Avoulou FL, Jerme MM, Ndanga EKK, Fekam FB. Hydroethanolic extracts of Erigeron floribundus and Azadirachta indica reduced Plasmodium berghei parasitemia in Balb/c mice. Evid Based Complement Alternat Med. 2018;2018:5156710. doi: 10.1155/2018/5156710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Akin-Osanaiye BC, Nok AJ, Ibrahim S, Inuwa HM, Onyike E, Amlabu E, et al. Antimalarial effect of neem leaves and neem stem barks extracts on Plasmodium berghei infected in the pathology and treatment of malaria. Int J Res Biochem Biophy. 2013;3:7–14. [Google Scholar]
- 146.Asrade S, Mengesha Y, Moges G, Gelayee DA. In vivo antiplasmodial activity evaluation of the leaves of Balanites rotundifolia (Van Tiegh) Blatter (Balanitaceae) against Plasmodium berghei. J Exp Pharmacol. 2017;9:59–66. doi: 10.2147/JEP.S130491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Otegbade OO, Ojo JA, Adefokun DI, Abiodun OO, Thomas BN, Ojurongbe O. Ethanol extract of Blighia sapida stem barks show remarkable prophylactic activity in experimental Plasmodium berghei–infected mice. Drug Target Insights. 2017;11:1177392817728725. doi: 10.1177/1177392817728725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Christian AG, Thecla EC, Dick EA, Chile AE, Chimsorom CK, Ckukwu ND, et al. In vivo antiplasmodial activity of Bombax buonopozense root barks aqueous extract in mice infected by Plasmodium berghei. J Tradit Chin Med. 2017;37:431–435. doi: 10.1016/S0254-6272(17)30148-6. [DOI] [PubMed] [Google Scholar]
- 149.Muluye AB, Melese E, Adinew GM. Antimalarial activity of 80 % methanolic extract of Brassica nigra (L.) Koch. (Brassicaceae) seeds against Plasmodium berghei infection in mice. BMC Compl Alternative Med. 2015;15:367. doi: 10.1186/s12906-015-0893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Eyasu M, Shibeshi W, Gida M. In vivo antimalarial activity of hydromethanolic leaf extract of Calpurnia aurea (Fabaceae) in mice infected with chloroquine-sensitive Plasmodium berghei. Int J Pharmacol. 2013;2:131–142. [Google Scholar]
- 151.Onaku LO, Attama AA, Okore VC, Tijani AY, Ngene AA, Esimone CO. Antagonistic antimalarial properties of pawpaw leaf aqueous extract in combination with artesunic acid in Plasmodium berghei-infected mice. J Vector Borne Dis. 2011;48:96–100. [PubMed] [Google Scholar]
- 152.Tona L, Mesia K, Ngimbi NP, Chrimwami B, Okondahoka, Cimanga K, et al. In vivo antimalarial activity of Cassia Occidentalism, Morinda morindoides and Phyllanthus niruri. Ann Trop Med Parasit. 2001;95:47–57. [PubMed]
- 153.Abdulrazak N, Asiya UI, Usman NS, Unata IM, Farida A. Anti-plasmodial activity of ethanolic extract of root and stem bark of Cassia sieberiana DC on mice. J Intercult Ethnopharmacol. 2015;4:96–101. doi: 10.5455/jice.20141231014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Adzu B, Abbah J, Vongtau H, Gamaniel K. Studies on the use of Cassia singueana in malaria ethnopharmacy. J Ethnopharmacol. 2003;88:261–267. doi: 10.1016/S0378-8741(03)00257-5. [DOI] [PubMed] [Google Scholar]
- 155.Jigam AA, Razaq UTA, Egbuta MN. In vivo antimalarial and toxicological evaluation of Chrozophora senegalensis A. Juss (Euphorbiaceae) extracts. J Appl Pharm Sci. 2011;1:90–4. [Google Scholar]
- 156.Ihekwereme CP, Okoye FK, Agu SC, Oli AN. Traditional consumption of the fruit pulp of Chrysophyllum albidum (Sapotaceae) in pregnancy may be serving as an intermittent preventive therapy against malaria infection. Anc Sci Life. 2017;36:191–195. doi: 10.4103/asl.ASL_208_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Okokon JE, Etebong EO, Udobang JA, Essien GE. Antiplasmodial and analgesic activities of Clausena anisate. Asian Pac J Trop Med. 2012;5:214–219. doi: 10.1016/S1995-7645(12)60027-3. [DOI] [PubMed] [Google Scholar]
- 158.Anato M, Ketema T. Anti-plasmodial activities of Combretum molle (Combretaceae) [Zwoo] seed extract in Swiss albino mice. BMC Res Notes. 2018;11:312. doi: 10.1186/s13104-018-3424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kweyamba PA, Zofou D, Efange N, Assob JN, Kitau J, Nyindo M. In vitro and in vivo studies on antimalarial activity of Commiphora africana and Dichrostachys cinerea used by the Maasai in Arusha region. Tanzania Malar J. 2019;18:119. doi: 10.1186/s12936-019-2752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Elufioye TO, Agbedahunsi JM. Antimalarial activities of Tithonia diversifolia (Asteraceae) and Crossopteryx febrifuga (Rubiaceae) on mice in vivo. J Ethnopharmacol. 2004;93:167–171. doi: 10.1016/j.jep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 161.Obey JK, Ngeiywa MM, Kiprono P, Omar S, vonWright A, Kauhanen J, et al. Antimalarial activity of Croton macrostachyus stem barks extracts against Plasmodium berghei in vivo. J Pathog. 2018;2018:393854. doi: 10.1155/2018/2393854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tona Ngimbi NP, Tsakala M, Mesia K, Cimanga K, Apers S, De Bruyne T, et al. Antimalarial activity of 20 crude extracts from nine African medicinal plants used in Kinshasa Congo. J Ethnopharmacol. 1999;68:193–203. doi: 10.1016/S0378-8741(99)00090-2. [DOI] [PubMed] [Google Scholar]
- 163.Mzena T, Swai H, Chacha M. Antimalarial activity of Cucumis metuliferus and Lippia kituiensis against Plasmodium berghei infection in mice. Res Rep Trop Med. 2018;9:81–88. doi: 10.2147/RRTM.S150091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Amelo W, Nagpal P, Makonnen E. Antiplasmodial activity of solvent fractions of methanolic root extract of Dodonaea angustifolia in Plasmodium berghei infected mice. BMC Complement Altern Med. 2014;14:462. doi: 10.1186/1472-6882-14-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ogbonna DN, Sokari TG, Agomuoh AA. Antimalarial activities of some selected traditional herbs from South Eastern Nigeria against Plasmodium species. Res J Parasit. 2008;3:25–31. doi: 10.3923/jp.2008.25.31. [DOI] [Google Scholar]
- 166.Gounoue Kamkumo R, Tsakem Nangap JM, Tchokouaha Yamthe LR, Ngueguim Tsofack F, Tsouh Fokou PV, Tchatat Tali MB, et al. Antimalarial activity of the aqueous extract of Euphorbia cordifolia Elliot in Plasmodium berghei-infected mice. Asian Pac J Trop Med. 2020;13:176–184. doi: 10.4103/1995-7645.280239. [DOI] [Google Scholar]
- 167.Oluwakanyinsola AS, Adeniyi YT, Babayi H, Angela CN, Anagbogu RA, Agbakwuru VA. Antimalarial activity of ethanolic stem barks extract of Faidherbia albida (Del) a Chev (Mimosoidae) in mice. Arch Appl Sci Res. 2010;2:261–8. [Google Scholar]
- 168.Nguta JM, Mbaria JM. Brine shrimp toxicity and antimalarial activity of some plants traditionally used in treatment of malaria in Msambweni district of Kenya. J Ethnopharmacol. 2013;148:988–992. doi: 10.1016/j.jep.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 169.Oluwatosin A, Tolulope A, Ayokulehin K, Okorie P, Aderemi K, Falade C, et al. Antimalarial potential of kolaviron, a biflavonoid from Garcinia kola seeds, against Plasmodium berghei infection in Swiss albino mice. Asian Pac J Trop Med. 2014;7:97–104. doi: 10.1016/S1995-7645(14)60003-1. [DOI] [PubMed] [Google Scholar]
- 170.Okokon JE, Ita BN, Udokpoh AE. The in vivo antimalarial activities of Uvariae chamae and Hippocratea africana. Ann Trop Med Parasitol. 2006;100:585–590. doi: 10.1179/136485906X118512. [DOI] [PubMed] [Google Scholar]
- 171.David-Oku E, Ifeoma OO, Christian AG, Dick EA. Evaluation of the antimalarial potential of Icacina senegalensis Juss (Icacinaceae) Asian Pac J Trop Med. 2014;7:S469–S472. doi: 10.1016/S1995-7645(14)60276-5. [DOI] [PubMed] [Google Scholar]
- 172.Birru EM, Geta M, Gurmu AE. Antiplasmodial activity of Indigofera spicata root extract against Plasmodium berghei infection in mice. Malar J. 2017;16:198. doi: 10.1186/s12936-017-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Onyeto CA, Akah PA, Nworu CS, Okoye TC, Okorie NA, Mbaoji FN, et al. Anti-plasmodial and antioxidant activities of methanol extract of the fresh leaves of Lophira lanceolata (Ochnaceae) Afr J Biotechnol. 2014;13:1731–1738. doi: 10.5897/AJB2014.13707. [DOI] [Google Scholar]
- 174.Christian AG, Akanimo EG, Ahunna AG, Nwakaego EM, Chimsorom CK. Antimalarial potency of the methanol leaf extract of Maerua crassifolia Forssk (Capparaceae) Asian Pac J Trop Dis. 2014;4:35–39. doi: 10.1016/S2222-1808(14)60310-8. [DOI] [Google Scholar]
- 175.Malebo HM, Tanja W, Cal M, Swaleh SAM, Omolo MO, Hassanali A, et al. Antiplasmodial, anti-trypanosomal, anti-leishmanial and cytotoxicity activity of selected Tanzanian medicinal plants. Tanzan J Health Res. 2015;4:226–234. doi: 10.4314/thrb.v11i4.50194. [DOI] [PubMed] [Google Scholar]
- 176.Okafor AI, Nok AJ, Inuwa HM. Antiplasmodial activity of aqueous leaf extract of Mucuna Pruriens Linn in mice infected with Plasmodium berghei (NK-65 Strain) J Appl Pharm Sci. 2013;3(4 Suppl 1):S52–S55. [Google Scholar]
- 177.Edagha IA, Peter AI, Aquaisua AN. Histopathological effect of Nauclea latifolia ethanolic leaf extract and artemether/lumefantrine on the hippocampus of P berghei-infected mice. Int J Brain Cognitive Sci. 2017;6:9–16. [Google Scholar]
- 178.Nworu CS, Ejikeme TI, Ezike AC, Ndu O, Akunne TC, Onyeto CA, et al. Anti-plasmodial and anti-inflammatory activities of cyclotide-rich extract and fraction of Oldenlandia affinis (R.& S.) D.C. (Rubiaceae) Afri Health Sci. 2017;17:827–43. doi: 10.4314/ahs.v17i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ramanitrahasimbola D, Rasoanaivo P, Ratsimamanga-Urverg S, Federici E, Palazzino G, Galeffi C, et al. Biological activities of the plant-derived bisindole voacamine with reference to malaria. Phytother Res. 2001;15:30–33. doi: 10.1002/1099-1573(200102)15:1<30::AID-PTR680>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 180.Ajala TO1, Igwilo CI, Oreagba IA, Odeku OA. The antiplasmodial effect of the extracts and formulated capsules of Phyllanthus amarus on Plasmodium yoelii infection in mice. Asian Pac J Trop Med. 2011;4:283–7. [DOI] [PubMed]
- 181.Ifeoma O, Samuel O, Itohan AM, Adeola SO. Isolation, fractionation and evaluation of the antiplasmodial properties of Phyllanthus niruri resident in its chloroform fraction. Asian Pac J Trop Med. 2013;6:169–175. doi: 10.1016/S1995-7645(13)60018-8. [DOI] [PubMed] [Google Scholar]
- 182.Adinew GM. Antimalarial activity of methanolic extract of Phytolacca dodecandra leaves against Plasmodium berghei infected Swiss albino mice. Int J Pharmacol Clin Sci. 2014;3:39–45. [Google Scholar]
- 183.Okokon JE, Antia BS, Igboasoiyi AC, Essien EE, Mbagwu HO. Evaluation of antiplasmodial activity of ethanolic seed extract of Picralima nitida. J Ethnopharmacol. 2007;111:464–467. doi: 10.1016/j.jep.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 184.Madara AA, Ajayi JA, Salawu OA, Tijani AY. Antimalarial activity of ethanolic leaf extract of Piliostigma thonningii Schum (Caesalpiniacea) in mice infected with Plasmodium berghei berghei. Afr J Biotechnol. 2010;9:3475–80. [Google Scholar]
- 185.Christian AG, Ahunna AG, Nwakaego EM, Chimsorom CK, Chile AE. Antimalarial potential of the ethanolic leaf extract of Pseudocedrala kotschyi. J Acute Dis. 2015;4:23–27. doi: 10.1016/S2221-6189(14)60077-9. [DOI] [Google Scholar]
- 186.Okokon JE, Ettebong E, Antia BS. In vivo antimalarial activity of ethanolic leaf extract of Stachytarpheta cayennensis. Indian J Pharmacol. 2008;40:111–113. doi: 10.4103/0253-7613.42303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Adegbolagun OM, Emikpe BO, Woranola IO, Ogunremi Y. Synergistic effect of aqueous extract of Telfaria occidentalis on the biological activities of artesunate in Plasmodium berghei infected mice. Afr Health Sci. 2013;13:970–976. doi: 10.4314/ahs.v13i4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Muregi FW, Ishih A, Miyase T, Suzuki T, Kino H, Amano T, et al. Antimalarial activity of methanolic extracts from plants used in Kenyan ethnomedicine and their interactions with chloroquine (CQ) against a CQ-tolerant rodent parasite, in mice. J Ethnopharmacol. 2007;111:190–195. doi: 10.1016/j.jep.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 189.Olanlokun JO, Oluwole MD, Afolayan AJ. In vitro antiplasmodial activity and prophylactic potentials of extract and fractions of Trema orientalis (Linn.) stem barks. BMC Complement Altern Med. 2017;17:407. doi: 10.1186/s12906-017-1914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fadare DA, Abiodun OO, Ajaiyeoba EO. In vivo antimalarial activity of Trichilia megalantha harms extracts and fractions in animal models. Parasitol Res. 2013;112:2991–2995. doi: 10.1007/s00436-013-3471-7. [DOI] [PubMed] [Google Scholar]
- 191.François G, Steenackers T, Timperman G, Aké Assi L, Haller RD, Bär S, et al. Retarded development of exoerythrocytic stages of the rodent malaria parasite Plasmodium berghei in human hepatoma cells by extracts from dioncophyllaceae and ancistrocladaceae species. Int J Parasitol. 1997;27:29–32. doi: 10.1016/S0020-7519(96)00171-3. [DOI] [PubMed] [Google Scholar]
- 192.Akuodor GC, Idris-Usman M, Anyalewechi N, Odo E, Ugwu CT, Akpan JL, et al. In vivo antimalarial activity of ethanolic leaf extract of Verbena hastata against Plasmodium berghei in mice. J Herb Med Toxicol. 2010;4:17–23. [Google Scholar]
- 193.Njan AA, Adzu B, Agaba AG, Byarugaba D, Díaz-Llera S, Bangsberg DR. The analgesic and antiplasmodial activities and toxicology of Vernonia amygdalina. J Med Food. 2008;11:574–581. doi: 10.1089/jmf.2007.0511. [DOI] [PubMed] [Google Scholar]
- 194.Iwalokun BA. Enhanced antimalarial effects of chloroquine by aqueous Vernonia amygdalina leaf extract in mice infected with chloroquine resistant and sensitive Plasmodium berghei strains. Afr Health Sci. 2008;8:25–35. [PMC free article] [PubMed] [Google Scholar]
- 195.Abosi AO, Raseroka BH. In vivo antimalarial activity of Vernonia amygdalina. Br J Biomed Sci. 2003;60:89–91. doi: 10.1080/09674845.2003.11783680. [DOI] [PubMed] [Google Scholar]
- 196.Dame ZT, Petros B, Mekonnen Y. Evaluation of anti-Plasmodium berghei activity of crude and column fractions of extracts from Withania somnifera. Turk J Biol. 2013;37:147–150. [Google Scholar]
- 197.Adugna M, Feyera T, Taddese W, Admasu P. In vivo antimalarial activity of crude extract of aerial part of Artemisia abyssinica against Plasmodium berghei in mice. Glob J Pharmacol. 2014;8:460–468. [Google Scholar]
- 198.Deressa T, Mekonnen Y, Animut A. In vivo antimalarial activities of Clerodendrum myricoides, Dodonea angustifolia, and Aloe debrana against Plasmodium berghei. Ethiop J Health Dev. 2010;24:25–29. [Google Scholar]
- 199.Muthaura CN, Rukunga GM, Chhabra SC, Omar SA, Guantai AN, Gathirwa JW, et al. Antimalarial activity of some plants traditionally used in treatment of malaria in Kwale district of Kenya. J Ethnopharmacol. 2007;112:545–551. doi: 10.1016/j.jep.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 200.Beha E, Jung A, Wiesner J, Rimpler H, Lanzer M, Heinrich M. Antimalarial activity of extracts of Abutilon grandiflorum G Don - a traditional Tanzanian medicinal plant. Phytother Res. 2004;18:236–40. doi: 10.1002/ptr.1393. [DOI] [PubMed] [Google Scholar]
- 201.Ajaiyeoba EO, Abiodun OO, Falade MO, Ogbole NO, Ashidi JS, Happi CT, et al. In vitro cytotoxicity studies of 20 plants used in Nigerian antimalarial ethnomedicine. Phytomedicine. 2006;13:295–298. doi: 10.1016/j.phymed.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 202.Adebayo JO, Balogun EA, Malomo SO, Soladoye AO, Olatunji LA, Kolawole OM, et al. Antimalarial activity of Cocos nucifera Husk fibre: further studes. Evid Based Complement Altern Med. 2013;2013:742476. doi: 10.1155/2013/742476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Falade MO, Akinboye DO, Gbotosho GO, Ajaiyeoba EO, Happi TC, Abiodun OO, et al. In vitro and in vivo antimalarial activity of Ficus thonningii Blume (Moraceae) and Lophira alata Banks (Ochnaceae), identified from the ethnomedicine of the Nigerian middle belt. J Parasit Res. 2014;2014:972853. doi: 10.1155/2014/972853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Iwalewa EO, Omisore NO, Adewunmi CO, Gbolade AA, Ademowo OG, Nneji C, et al. Anti-protozoan activities of Harungana madagascariensis stem barks extract on trichomonads and malaria. J Ethnopharmacol. 2008;117:507–511. doi: 10.1016/j.jep.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 205.Gathirwa JW, Rukunga GM, Njagi EN, Omar SA, Mwitari PG, Guantai AN, et al. The in vitro anti-plasmodial and in vivo antimalarial efficacy of combinations of some medicinal plants used traditionally for treatment of malaria by the Meru community in Kenya. J Ethnopharmacol. 2008;115:223–231. doi: 10.1016/j.jep.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 206.Simelane MBC, Shonhai A, Shode FO, Smith P, Singh M, Opoku AR. Anti-plasmodial activity of some Zulu medicinal plants and of some triterpenes isolated from them. Molecules. 2013;18:12313. doi: 10.3390/molecules181012313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Steele JC, Warhurst DC, Kirby GC, Simmonds MSJ. In vitro and in vivo evaluation of betulinic acid as an antimalarial. Phytother Res. 1999;13:115–119. doi: 10.1002/(SICI)1099-1573(199903)13:2<115::AID-PTR404>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 208.Builders MI, Wannang NN, Ajoku GA, Builders PF, Orisadipe A, Aguiyi JC. Evaluation of the antimalarial potential of Vernonia ambigua Kotschy and Peyr (Asteraceae) Int J Pharmacol. 2011;7:238–247. doi: 10.3923/ijp.2011.238.247. [DOI] [Google Scholar]
- 209.Ngbolua KN, Rakotoarimanana H, Rafatro H, Ratsimamanga US, Mudogo V, Mpiana PT, et al. Comparative antimalarial and cytotoxic activities of two Vernonia species: Vernonia amygdalina from the Democratic Republic of Congo and Vernonia cinerea subsp vialis endemic to Madagascar. Int J Biol Chem Sci. 2011;5:345–353. [Google Scholar]
- 210.Benoit-Vical F, Imbert C, Bonfils JP, Sauvaire Y. Antiplasmodial and antifungal activities of iridal, a plant triterpenoid. Phytochemistry. 2003;62:747–751. doi: 10.1016/S0031-9422(02)00625-8. [DOI] [PubMed] [Google Scholar]
- 211.Applequist WL, Ratsimbason M, Kuhlman A, Ratonandrasana S, Rasamison V, Kingston DG. Antimalarial use of Malagasy plants is poorly correlated with performance in antimalarial bioassays. Econ Bot. 2017;71:75–82. doi: 10.1007/s12231-017-9373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Thiengsusuk A, Chaijaroenkul W, NaBangchang K. Antimalarial activities of medicinal plants and herbal formulations used in Thai traditional medicine. Parasitol Res. 2013;112:1475. doi: 10.1007/s00436-013-3294-6. [DOI] [PubMed] [Google Scholar]
- 213.Udobang JA, Nwafor PA, Okokon JE. Analgesic and antimalarial activities of crude leaf extract and fractions of Acalypha wilkensiana. J Ethnopharmacol. 2010;127:373–378. doi: 10.1016/j.jep.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 214.Mohammed T, Erko B, Giday M. Evaluation of antimalarial activity of leaves of Acokanthera schimperi and Croton macrostachyus against Plasmodium berghei in Swiss albino mice. Compl Altern Med. 2014;14:314. doi: 10.1186/1472-6882-14-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kuria KA, De Coster S, Muriuki G, Masengo W, Kibwage I, Hoogmartens J, et al. Antimalarial activity of Ajuga remota Benth (Labiatae) and Caesalpinia Volkensii Harms (Caesalpiniaceae): in vitro confirmation of ethnopharmacological use. J Ethnopharmacol. 2001;74:141–148. doi: 10.1016/S0378-8741(00)00367-6. [DOI] [PubMed] [Google Scholar]
- 216.Mesfin A, Giday M, Animut A, Teklehaymanot T. Ethnobotanical study of antimalarial plants in Shineile District, Somali Region, Ethiopia, and in vivo evaluation of selected ones against Plasmodium berghei. J Ethnopharmacol. 2012;139:221–227. doi: 10.1016/j.jep.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 217.Hilou A, Nacoulma OG, Guiguemde TR. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J Ethnopharmacol. 2006;103:236–240. doi: 10.1016/j.jep.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 218.Adebajo AC, Odediran SA, Aliyu FA, Nwafor PA, Nwoko NT, Umana US. In vivo antiplasmodial potentials of the combinations of four Nigerian antimalarial plants. Molecules. 2014;19:13136–13146. doi: 10.3390/molecules190913136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dikasso D, Mekonnen E, Debella A, Abebe D, Urga K, Menonnen W, et al. In vivo antimalarial activity of hydroalcoholic extracts from Asparagus africanus Lam in mice infected with Plasmodium berghei. Ethiop J Health Dev. 2006;20:112–8. [Google Scholar]
- 220.Yerbanga RS, Lucantoni L, Lupidi G, Dori GU, Tepongning NR, Nikiéma JB, et al. Antimalarial plant remedies from Burkina Faso: their potential for prophylactic use. J Ethnopharmacol. 2012;140:255–260. doi: 10.1016/j.jep.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 221.Karou SD, Tchacondo T, Ouattara L, Anani K, Savadogo A, Agbonon A, et al. Antimicrobial, antiplasmodial, haemolytic and antioxidant activities of crude extracts from three selected Togolese medicinal plants. Asian Pac J Trop Med. 2011;4:808–813. doi: 10.1016/S1995-7645(11)60199-5. [DOI] [PubMed] [Google Scholar]
- 222.Bonkian LN, Yerbanga RS, Koama B, Soma A, Cisse M, Valea I, et al. In vivo antiplasmodial activity of two Sahelian plant extracts on Plasmodium berghei ANKA infected NMRI mice. Evid Based Complement Alternat Med. 2018;24:6859632. doi: 10.1155/2018/6859632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ajaiyeoba E, Ashidi J, Abiodun O, Okpako L, Ogbole O, Akinboye D, et al. Antimalarial ethnobotany: in vitro antiplasmodial activity of seven plants indentified in the Nigerian middle belt. Pharm Biol. 2005;42:588–591. doi: 10.1080/13880200490902455. [DOI] [Google Scholar]
- 224.Kefe A, Giday M, Mamo H, Erko B. Antimalarial properties of crude extracts of seeds of Brucea antidysenterica and leaves of Ocimum lamiifolium. BMC Complement Altern Med. 2016;16:118. doi: 10.1186/s12906-016-1098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Innocent E, Moshi MJ, Masimba PJ, Mbwambo ZH, Kapingu MC, Kamuhabwa A. Screening of traditionally used plants for in vivo antimalarial activity in mice. Afr J Tradit Complement Altern Med. 2009;6:163–167. doi: 10.4314/ajtcam.v6i2.57088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mengiste B, Mekonnen E, Urga K. In vivo animalarial activity of Dodonaea angustifolia seed extracts against Plasmodium berghei in mice model. MEJS. 2012;4:147–163. doi: 10.4314/mejs.v4i1.74056. [DOI] [Google Scholar]
- 227.Biruksew A, Zeynudin A, Alemu Y, Golassa L, Yohannes M, Debella A, et al. Zingiber officinale Roscoe and Echinops kebericho Mesfin showed antiplasmodial activities against Plasmodium berghei in a dose-dependent manner in Ethiopia. Ethiop J Health Sci. 2018;28:655. doi: 10.4314/ejhs.v28i5.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Agbaje EO, Onabanjo AO. The effects of extracts of Enantia chlorantha in malaria. Ann Trop Med Parasitol. 1991;85:585–590. doi: 10.1080/00034983.1991.11812613. [DOI] [PubMed] [Google Scholar]
- 229.Ajayi EIO, Adelekeb MA, Adewumia TY, Adeyemia AA. Antiplasmodial activities of ethanol extracts of Euphorbia hirta whole plant and Vernonia amygdalina leaves in Plasmodium berghei-infected mice. J Taibah Univ Sci. 2017;11:831–835. doi: 10.1016/j.jtusci.2017.01.008. [DOI] [Google Scholar]
- 230.Omole AR, Malebo MH, Nondo SOR, Katani S, Mbugi H, Midiwo J, et al. In vivo anti-plasmodial activity of crude extracts of three medicinal plants used traditionally for malaria treatment in Kenya. Eur J Med Plants. 2018;24:1–7. doi: 10.9734/EJMP/2018/42874. [DOI] [Google Scholar]
- 231.Nureye D, Assefa S, Nedi T, Engidawork E. In vivo antimalarial activity of the 80% methanolic root barks extract and solvent fractions of Gardenia ternifolia Schumach & Thonn (Rubiaceae) against Plasmodium berghei. Evid Based Complement Alternat Med. 2018;2018:9217835. doi: 10.1155/2018/9217835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Beaufay C, Hérent MF, Quetin-Leclercq J, Bero J. In vivo antimalarial activity and toxicity studies of triterpenic esters isolated from Keetia leucantha and crude extracts. Malar J. 2017;16:406. doi: 10.1186/s12936-017-2054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bankole AE, Adekunle AA, Sowemimo AA, Umebese CE, Abiodun O, Gbotosho GO. Phytochemical screening and in vivo antimalarial activity of extracts from three medicinal plants used in malaria treatment in Nigeria. Parasitol Res. 2016;115:299–305. doi: 10.1007/s00436-015-4747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jansen O, Tchinda AT, Loua J, Esters V, Cieckiewicz E, Ledoux A. Antiplasmodial activity of Mezoneuron benthamianum leaves and identification of its active constituents. J Ethnopharmacol. 2017;203:20–26. doi: 10.1016/j.jep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 235.Udobre AS, Udobang JA, Udoh AE, Anah VU, Akpan AE, Charles GE. Effect of methanol leaf extract of Nauclea latifolia on albino mice infected with Plasmodium berghei berghei. Afr J Pharmacol Ther. 2013;2:83–87. [Google Scholar]
- 236.Mesia K, Tona L, Mampunza MM, Ntamabyaliro N, Muanda T, Muyembe T, et al. Antimalarial efficacy of a quantified extract of Nauclea pobeguinii stem barks in human adult volunteers with diagnosed uncomplicated falciparum malaria .Part 2: a clinical phase IIB trial. Planta Med. 2012;78:853–60. doi: 10.1055/s-0031-1280359. [DOI] [PubMed] [Google Scholar]
- 237.Okeola VO, Adaramoye OA, Nneji CM, Falade CO, Farombi EO, Ademowo OG. Antimalarial and antioxidant activities of methanolic extract of Nigella sativa seeds (black cumin) in mice infected with Plasmodium yoelii nigeriensis. Parasitol Res. 2011;108:1507–1512. doi: 10.1007/s00436-010-2204-4. [DOI] [PubMed] [Google Scholar]
- 238.Girma S, Giday M, Erko B, Mamo H. Effect of crude leaf extract of Osyris quadripartita on Plasmodium berghei in Swiss albino mice. BMC Complement Alternative Med. 2015;15:184. doi: 10.1186/s12906-015-0715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kabiru AY, Ibikunle GF, Innalegwu DA, Bola BM, Madaki FM. In vivo antiplasmodial and analgesic effect of crude ethanol extract of Piper guineense leaf extract in Albino Mice. Scientifica (Cairo). 2016: 8687313. [DOI] [PMC free article] [PubMed]
- 240.Hiben MG, Sibhat GG, Fanta BS, Gebrezgi HD, Tesema SB. Evaluation of Senna singueana leaf extract as an alternative or adjuvant therapy for malaria. J Tradit Complement Med. 2015;6:112–117. doi: 10.1016/j.jtcme.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tadesse SA, Wubneh ZB. Antimalarial activity of Syzygium guineense during early and established Plasmodium infection in rodent models. BMC Complement Alternative Med. 2017;17:21. doi: 10.1186/s12906-016-1538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Adepiti AO, Iwalewa EO. Evaluation of the combination of Uvaria chamae (P Beauv) and amodiaquine in murine malaria. J Ethnopharmacol. 2016;193:30–5. doi: 10.1016/j.jep.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 243.Masaba SC. The antimalarial activity of Vernonia amygdalina Del (Compositae) Trans R Soc Trop Med Hyg. 2000;94:694–695. doi: 10.1016/S0035-9203(00)90236-0. [DOI] [PubMed] [Google Scholar]
- 244.Omoregie ES, Pal A. Antiplasmodial, antioxidant and immunomodulatory activities of ethanol extract of Vernonia amygdalina del. leaves in Swiss mice. Avicenna J Phytomed. 2016;6:236–7. [PMC free article] [PubMed] [Google Scholar]
- 245.Challand S, Willcox M. A Clinical trial of the traditional medicine Vernonia amygdalina in the treatment of uncomplicated Malaria. J Altern Complement Med. 2009;15:1231–1237. doi: 10.1089/acm.2009.0098. [DOI] [PubMed] [Google Scholar]
- 246.Ajayi BB, Ogunsola JO, Olatoye OI, Antia RE, Agbedea S. Effects of pituitary extract, ovaprim, and bitter leaves (Vernonia amygdalina) on the histopathology of African catfish (Clarias gariepinus) Aquacult. Fish. 2018;3:232–237. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. In vitro and in vivo studies reporting inactive antiplasmodial or antimalarial activity. Table S2. List of active compounds identified from plants.
Data Availability Statement
Not applicable.