Abstract
Difficulties in the collection of hematopoietic stem and progenitor cells (HSPCs) from Fanconi anemia (FA) patients have limited the gene therapy in this disease. We have investigated (ClinicalTrials.gov, NCT02931071) the safety and efficacy of filgrastim and plerixafor for mobilization of HSPCs and collection by leukapheresis in FA patients. Nine of eleven enrolled patients mobilized beyond the threshold level of 5 CD34+ cells/μL required to initiate apheresis. A median of 21.8 CD34+ cells/μL was reached at the peak of mobilization. Significantly, the oldest patients (15 and 16 years old) were the only ones who did not reach that threshold. A median of 4.27 million CD34+ cells/kg was collected in 2 or 3 aphereses. These numbers were markedly decreased to 1.1 million CD34+ cells/kg after immunoselection, probably because of weak expression of the CD34 antigen. However, these numbers were sufficient to facilitate the engraftment of corrected HSPCs in non-conditioned patients. No procedure-associated serious adverse events were observed. Mobilization of CD34+ cells correlated with younger age, higher leukocyte counts and hemoglobin values, lower mean corpuscular volume, and higher proportion of CD34+ cells in bone marrow (BM). All these values offer crucial information for the enrollment of FA patients for gene therapy protocols.
Keywords: Fanconi anemia, HSPC collection, gene therapy, Mozobil, mobilization, leukapheresis, plerixafor, AMD3100, lentiviral vector, filgrastim
Graphical abstract
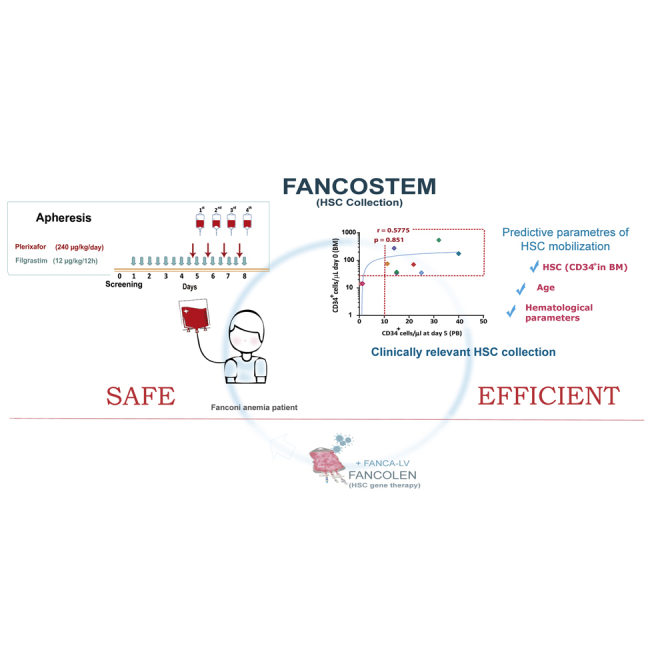
Mobilization and collection of HSPCs from FA patients with sufficient HSPC reserve is a safe and efficient procedure, incorporating filgrastim and plerixafor as mobilization agents. Adequate HSPC mobilization correlates with younger age, higher leukocyte counts and hemoglobin values, lower mean corpuscular volume, and higher BM CD34+ cell numbers.
Introduction
Current hematopoietic gene therapy protocols rely on the ex vivo genetic modification of autologous hematopoietic stem and progenitor cells (HSPCs) with either gammaretroviral or lentiviral vectors and, more recently, with gene editing constructs. Corrected cells are then re-infused into affected patients, in most cases after conditioning with cytotoxic drugs (see reviews by Naldini1 and Morgan et al.2). The collection of clinically relevant numbers of HSPCs thus constitutes an initial critical step in the development of efficient hematopoietic gene therapy protocols.
In most of the hematopoietic diseases in which gene therapy has been evaluated to date, phenotypic defects are evident only in terminally differentiated cells or in committed progenitors but not in hematopoietic stem cells (HSCs). This implies that collection of significant HSPC quantities does not generally constitute a limiting factor in the gene therapy of affected patients.3,4 In patients with bone marrow failure (BMF) syndromes, the situation can be markedly different. In the particular case of Fanconi anemia (FA), it has been shown that FA genes are ubiquitously expressed not only in mature cells or in committed progenitor cells but also in primitive HSCs.5,6 Moreover, because FA proteins participate in different pathways involved in cell proliferation, differentiation, and survival (see reviews in Ceccaldi et al.7 and Bagby8), reduced numbers of HSPCs have been observed in bone marrow (BM) of FA patients.9,10 Related to these observations, previous FA clinical trials have found serious obstacles to the collection of significant CD34+ cell numbers from either BM or peripheral blood (PB) utilizing granulocyte colony stimulating factor (G-CSF),10, 11, 12, 13 confirming the importance of adequate HSPC collection as a critical step in FA gene therapy.
The combination of potent mobilization agents, specifically filgrastim and plerixafor, has enabled enhanced potential of HSPC collection from patients with poor HSC reserve.14 Our previous studies have shown that FA CD34+ cells mobilized to PB with these agents, and then genetically corrected via lentiviral transduction, can progressively engraft in immunodeficient mice15 and also, remarkably, non-conditioned FA patients.16 These studies demonstrated the efficacy of these drugs in the mobilization of hematopoietic repopulating cells in patients with FA.
Although HSC mobilization with filgrastim and plerixafor is now frequently used for the collection of HSPCs in gene therapy protocols, the safety and efficacy of the collection of HSPCs mobilized with these drugs in patients with FA had never been previously investigated. Here we report the results of the first clinical trial in which a specific mobilization regimen with filgrastim and plerixafor was used to collect CD34+ cells from FA patients. The proposed HSC mobilization with these agents should facilitate progressive development of safe and efficient protocols of FA gene therapy.
Results
Patient characteristics
Thirteen FA-A patients, aged 3–16 years, were enrolled. Two patients were ineligible because the diepoxybutane (DEB) test showed that the proportion of PB T cells with chromosomal aberrations (24% and 5%, respectively) was below the threshold of 50% frequently utilized to distinguish FA patients from those with somatic mosaicism.17 The remaining eleven patients were finally included in the trial and received the HSPC mobilization treatment. The main characteristics of these patients are shown in Table 1. All these patients showed a high proportion of PB T lymphocytes with chromosomal breaks upon in vitro exposure to DEB, consistent with a characteristic FA diagnosis (see Table 1). BM colony forming cells (CFCs) were hypersensitive to mitomycin C (MMC). Only in the case of patient 01001 was a survival of 11.8% to 10 nM MMC observed, although no other parameters were consistent with somatic mosaicism in this patient (data not shown).
Table 1.
Main characteristics of Fanconi anemia patients enrolled in the FANCOSTEM-I clinical trial
| Code | Sex | Ethnicity | Age (years) | Weight (kg) | % PB T cells with Chr breaks (DEB) | Compl. group | BM CFC survival (10 nM MMC) | Mutation 1 (FANCA) | Mutation 2 (FANCA) |
|---|---|---|---|---|---|---|---|---|---|
| 01001 | male | European/white | 15 | 65.0 | 84 | FA-A | 11.8 | Del exons 19–22 | c.3348+A>G (intronic region exon 33) |
| 01002 | male | Roma/gypsy | 16 | 42.0 | 84 | FA-A | ND | c.295C>T (exon 4) | c.295C>T (exon 4) |
| 01003 | male | Roma/gypsy | 3 | 10.0 | 64 | FA-A | 0.0 | c.295C>T (exon 4) | c.295C>T (exon 4) |
| 01005 | male | Roma/gypsy | 5 | 16.0 | 80 | FA-A | 0.0 | c.295C>T (exon 4) | c.295C>T (exon 4) |
| 02002 | male | Roma/gypsy | 3 | 17.0 | 80 | FA-A | 0.0 | c.295C>T (exon 4) | c.295C>T (exon 4) |
| 02003 | male | European/white | 3 | 9.0 | 66 | FA-A | 0.0 | c.1858_1859insC (exon 21) | c.893+2T>C (intron 10) |
| 02004 | male | European/white | 6 | 14.0 | 66 | FA-A | 3.9 | c.1115_1118delTTGG (exon 13) | c.1115_1118delTTGG (exon 13) |
| 02005 | male | Roma/gypsy | 4 | 17.0 | 74 | FA-A | 0.0 | c.295C>T | c.295C>T |
| 02006 | male | European/white | 5 | 13.9 | 64 | FA-A | 0.0 | c.3788_3790delTCT (exon 38) | c.2851C>G (exon 29) |
| 02007 | female | European/white | 7 | 18.0 | 72 | FA-A | 0.0 | c.4130C>G exon 41 | c.1115_1118delTTGG (exon 13) |
| 02008 | male | Middle Eastern | 4 | 12.7 | 100 | FA-A | 0.0 | c.3788_3790delTCT | arr[hg19]16q24.3(89,804,013-89,896,981)x1 |
Basal PB parameters at screening (visit 0) determined in each of the eleven patients enrolled in the clinical trial are shown in Figure S1a. Although the inclusion criteria indicated that patients should fulfill at least two of the following criteria, hemoglobin (Hb) ≥ 8 g/dL, neutrophils ≥ 0.75 × 109 cells/L, and platelets ≥ 30 × 109 cells/L (see gray lines in Figure S1a), all patients, with the exception of patient 02008, fulfilled all three criteria.
Aliquots of BM aspirates conducted at visit 0 were used to analyze the content of CD34+ cells, CD34+/CD38− cells, and CFCs. As shown in Figure S1b, patients 02002 and 02005 were those with the highest proportion of CD34+ cells (1.44% and 1.38%, respectively) and total concentration of CD34+ cells in BM (545 and 276 CD34+ cells/μL BM, respectively). Compared to BM samples from healthy donors (HDs), the intensity of expression of the CD34 antigen in BM cells from patients included in the trial was significantly lower (see mean fluorescence intensity [MFI] values in Figure S2), suggesting their reduced content in primitive HSCs.18,19
Safety studies
Patient 01002 was treated with filgrastim and the four doses of plerixafor that permitted the clinical trial. Nevertheless, no apheresis procedures could be performed in this patient since CD34+ cell counts never reached the threshold of 5 CD34+ cells/μL PB. Patient 01001 did not receive the 4th plerixafor dose because of the inefficacy of the previous three doses. Among the other nine patients, four patients received three doses of plerixafor and underwent three apheresis procedures, whereas the other five patients received two doses of plerixafor and two aphereses were carried out. Decisions to continue mobilization, and the subsequent leukapheresis procedures, were based on a careful risk/benefit assessment for each patient.
Twenty-two adverse events (AEs) were observed throughout the study. However, no serious adverse events (SAEs) related to the procedure have been observed in any of the eleven patients who received the HSPC mobilization regimen. All patients but one required platelet transfusion (median of 1 platelet transfusion; range 0–2), either for catheter placement or during intervals between the leukapheresis procedures. Additionally, all patients who underwent leukapheresis received at least one packed red blood cell transfusion (median 3, range 1–4). A summary of the AEs is shown in Table 2. Six patients (55.5%) reported drug-related AEs previously associated with filgrastim or plerixafor administration. The most frequent of these were abdominal pain, vomiting, and fever, each of which was reported in two patients. When all AEs were considered (not only those related to mobilization agents), abdominal pain and pain at the catheter site were the most frequent ones. All but one of the AEs were mild and have been frequently associated with these agents and with HSPC collection procedures in children.20 Of interest, none of the collection procedures had to be prematurely interrupted because of patient AEs. The median number of blood volumes processed on the first, second, and third collection days were 4.25 (3.25–5.19), 4.88 (3.25–6.07), and 3.79 (3.33–4.86), respectively (Table S1). Although these blood volume numbers were quite large, no clinically relevant AEs related to metabolic changes or anticoagulation were observed.
Table 2.
Summary of the adverse events of the FANCOSTEM clinical trial
| N (patients) | % | |
|---|---|---|
| Drug-related AEs | ||
| Any drug-related adverse event | 7 | 63.6 |
| Abdominal pain | 2 | 18.2 |
| Vomiting | 2 | 18.2 |
| Fever | 2 | 18.2 |
| Nausea | 1 | 9.1 |
| Local pain at administration site | 1 | 9.1 |
| Anemia | 1 | 9.1 |
| Pruritus | 1 | 9.1 |
| Total AEs | ||
| Any adverse event | 9 | 81.8 |
| Abdominal pain | 3 | 27.3 |
| Pain at central venous line access | 3 | 27.3 |
| Fever | 2∗∗ | 18.2 |
| Vomiting | 2 | 18.2 |
| Anemia | 2∗ | 18.2 |
| Tachycardia | 2 | 18.2 |
| Nausea | 1 | 9.1 |
| Pain at administration site | 1 | 9.1 |
| Bleeding at central venous line access | 1 | 9.1 |
| Throat pain | 1 | 9.1 |
| Pleural effusion | 1 | 9.1 |
| Hypotension | 1 | 9.1 |
| Pruritus | 1 | 9.1 |
| Headache | 1 | 9.1 |
The table summarizes both the filgrastim- and/or plerixafor-related adverse events as well as the total adverse events. Adverse events are reported as the number and percentage of patients affected. Patients could experience more than one adverse event.
All adverse events were graded mild except: ∗one moderate and the other one severe; ∗∗one case graded moderate.
Blood cell counts and Hb levels determined during the 1 year of follow-up, and also prior to the recruitment, are presented in Figure S3. Because three patients were withdrawn from the FANCOSTEM-I trial immediately after CD34+ cell collection in order to receive treatment on the accompanying gene therapy clinical study, this figure represents the six patients who completed the mobilization, collection, and follow-up in the absence of infusion of gene-corrected HSPCs. As shown in this figure, no evident changes in any of the PB parameters were observed as a consequence of collection of mobilized HSCs. Moreover, no statistical differences in any of these parameters or in the number of BM CD34+ cells were observed at the end of the follow-up period compared to analyses performed at screening (data not shown). Cytogenetic studies as well as comparative genomic hybridization (array CGH) and next-generation sequencing analysis (NGS) of a myeloid malignancy-associated gene panel were performed at screening visit and at the end of the follow-up. A BCOR variant below the usual threshold of detection was detected at screening visit for patient 02008. None of the studies mentioned above showed significant abnormalities 12 months after mobilization in this series.
Analysis of the efficacy of filgrastim and plerixafor to mobilize HSPCs in FA patients
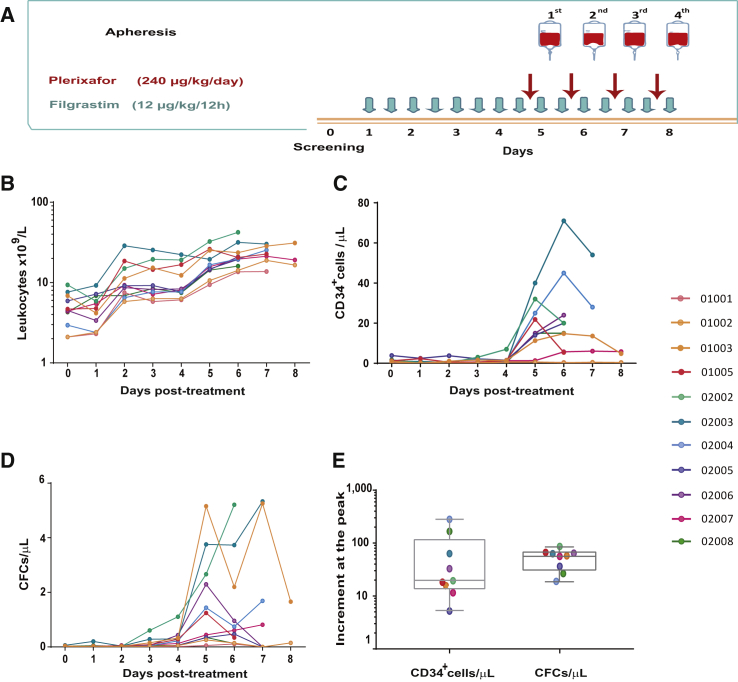
The HSC mobilization protocol shown in Figure 1A induced a progressive increase in PB leukocytes subsequent to the first administration of filgrastim (Figure 1B). More significantly, in nine out of the eleven treated patients the combined administration of filgrastim and plerixafor mobilized ≥5 CD34+ cells/μL PB (Figure 1C). The only two patients who did not mobilize threshold CD34+ cell numbers (01001 and 01002) were 15- and 16-year-old patients (respective weights 65 and 42 kg). This contrasts with all the other patients, with ages of 3–7 years (weight range: 9.2–17.6 kg), who mobilized to a median PB level of 21.8 CD34+ cells/μL at the peak of mobilization. As shown in Figure 1D, significant numbers of CFCs also were mobilized after administration of filgrastim and plerixafor. To evaluate more directly the efficacy of the proposed mobilization regimen in FA patients, the fold increase of CD34+ cells and CFCs at the peak of the mobilization (with respect to basal numbers determined at visit 0) was also represented. As shown in Figure 1E, median fold increases of CD34+ cells and CFCs were 19.4 and 57.3, respectively.
Figure 1.
HSPC mobilization induced by filgrastim and plerixafor in patients with Fanconi anemia
(A) Schematic representation of the CD34+ cell mobilization protocol based on the subcutaneous injection of filgrastim (twice daily; 12 μg/kg/12 h for up to 8 days) and plerixafor (240 μg/kg body weight/day; up to 4 doses). After each administration of plerixafor, CD34+ cell numbers were determined, and aphereses were initiated when numbers were higher than 5 CD34+ cells/μL PB. (B–D) kinetics of PB leukocytes, CD34+ cells, and CFCs, respectively, at different time points after administration of the mobilizing drugs. (E) increment of CD34+ cells and CFCs at the peak of mobilization compared to basal numbers determined at visit 0. Data corresponding to each of the treated patients are represented by a different color according to the ID indicated in the figure.
Collection of mobilized HSCs from FA patients treated with filgrastim and plerixafor
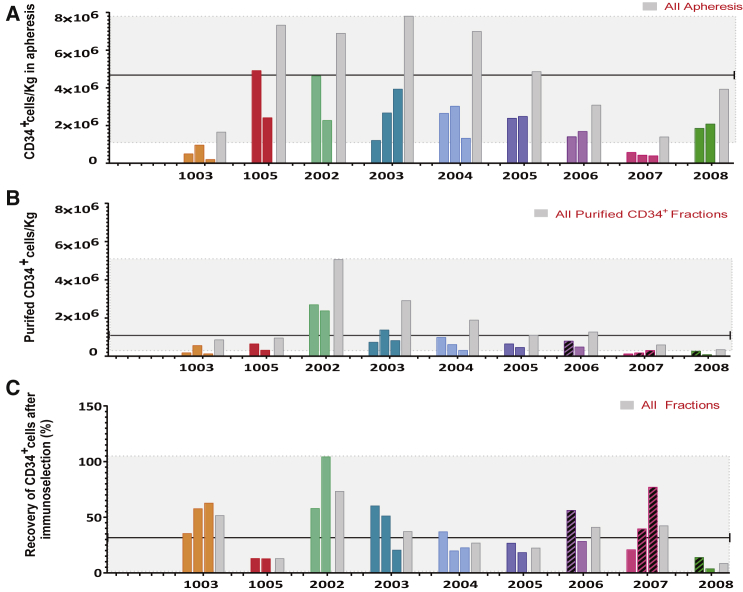
Numbers of CD34+ cells collected in each of the 22 apheresis procedures carried out in the nine patients who mobilized ≥5 CD34+ cells/μL PB are represented in Figure 2A. The total number of CD34+ cells collected in the different apheresis procedures reached a median value of 4.86 × 106 CD34+ cells/kg (1.39–7.81 × 106 CD34+ cells/kg) (Table S2). As happened in BM, mobilized PB (mPB) CD34+ cells showed a low intensity of expression of the CD34 antigen (Figure S2b). The cellularity corresponding to the first apheresis procedure was higher compared to the second and third ones. However, when the proportions of HSPCs were considered, no statistical differences were found among the different apheresis procedures (Figure S4).
Figure 2.
Analysis of the CD34+ cell content in apheresis and immunoselection products after HSPC mobilization with filgrastim and plerixafor
(A) Number of CD34+ cells/kg collected in each of the apheresis procedures from the nine patients who fulfilled apheresis criteria. Total number of collected CD34+ cells/kg corresponding to each patient are also shown (gray bars). (B) CD34+ cell numbers after immunoselection. (C) CD34+ cell recovery after immunoselection. Dashed bars in (B) and (C) represent data corresponding to immunoselection processes modified to improve the CD34+ cell yields. Black lines in each panel represent median values and shadow areas the interval range of the median. Patients’ color codes are the same as in Figure 1.
Immunoselection of PB HSPCs after mobilization with filgrastim and plerixafor
Numbers of CD34+ cells obtained in the immunoselected samples were on average 4 times lower than numbers corresponding to the apheresis collections (Figure 2B), a median value of 1.10 × 106 CD34+ cells/kg (range 0.34 to 5.06 × 106 CD34+ cells/kg) compared to the median value of 4.27 × 106 CD34+ cells/kg obtained prior to immunoselection. This low yield of CD34+ cells obtained in the immunoselection process was associated to the low expression of the CD34 antigen in FA CD34+ cells (Figure S2b). CD34+ cell purities and other characteristics of the different immunoselected products are shown in Figure S5. In terms of purity, the median percentage of CD34+ cells was 25.3%, with a wide range of 4.2% to 80.0% due to the different purification processes used in four immunoselection processes (see Supplemental materials and methods).
The recovery of CD34+ cells after each immunoselection process is represented in Figure 2C. As shown in the figure, the median recovery of CD34+ cells after immunoselection was 32.0% (range 3.8% to 104.5%). Because in some cases standard CliniMACS purifications resulted in very poor CD34+ cell recoveries in four immunoselection procedures (dashed bars in Figures 2B and 2C), the cell washing process that follows the attachment of CD34+ cells to the magnetic column was modified (see Supplemental materials and methods). As shown in Table S3, the recovery of CD34+ cells obtained in the modified immunoselections increased 2.9-fold over recovery corresponding to conventional immunoselections, although this occurred at the expense of a 6.6-fold decrease in the CD34+ cell purity.
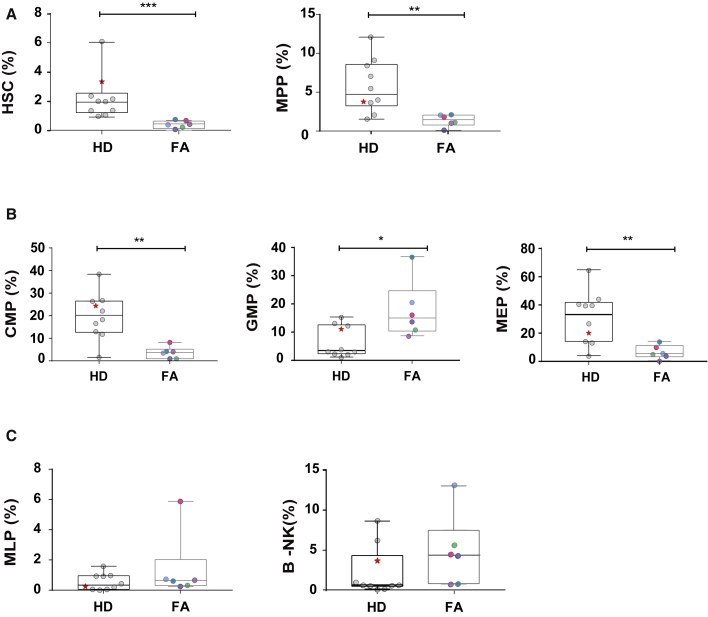
To investigate whether low levels of CD34 expression (Figure S2) were associated with a lower primitive HSC content in FA CD34+ cells, purified mPB CD34+ cells from six FA patients and ten HDs (treated either with filgrastim alone or with filgrastim and plerixafor) were investigated (see Supplemental materials and methods). In all instances the same gates were used for the analysis of FA and HD samples (see a representative analysis in Figure S6). Compared to mPB CD34+ cells from HDs, mPB CD34+ cells from FA patients contained significantly lower numbers of primitive HSCs and multipotent (MPP) precursors (4.2–5.6 fold, respectively; Figure 3A). Additionally, a significant decrease was observed in the content of committed myeloid (CMP) and myeloid-erythroid (MEP) progenitors, while a significant increase was observed in the granulocyte-monocyte progenitors (GMP) (Figure 3B). No significant differences were observed in the more differentiated multi-lymphoid progenitor (MLP) and B lymphocytes-natural killer (B-NK) progenitors (Figure 3C).
Figure 3.
Analysis of different HSPC populations in mobilized PB CD34+ cells corresponding to FA patients and healthy donors
The figure shows the content of HSCs (A) and more differentiated progenitor cells (B and C) in mPB CD34+ cells from nine HDs and six FA patients. FA patients were pre-treated with filgrastim and plerixafor (color codes as in Figure 1). HDs were treated only with filgrastim or with plerixafor and filgrastim (marked with a star). ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001.
Exploratory studies
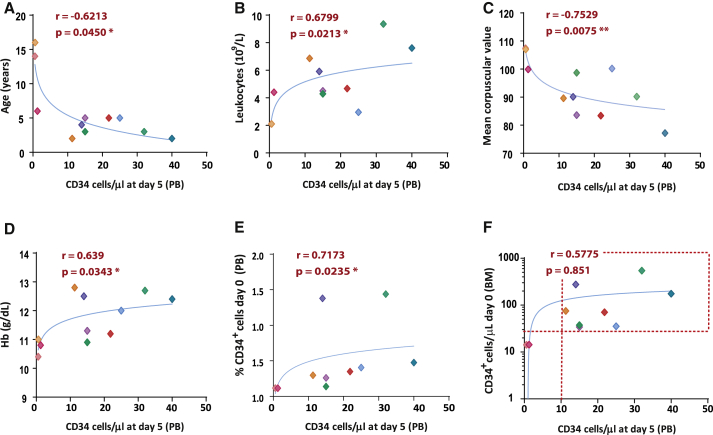
In these studies, we investigated variables associated with the efficacy of HSPC mobilization, defined by CD34+ cell numbers either at day 5 of mobilization (Figure 4) or at the peak of mobilization (Figure S7). In these analyses we observed that efficient CD34+ cell mobilization was associated with younger age, higher leukocyte counts and hemoglobin values, as well as low mean corpuscular volumes (MCVs), and also with a higher proportion of CD34+ cells in BM at screening. Also of significance is the fact that every patient with at least 30 CD34+ cells/μL BM at screening mobilized to levels > 10 CD34+ cells/μL PB. MCV was the only variable that remained statistically significant in a multivariate analysis: β −0.9 (−1.6 to −0.16) (p = 0.02). Additionally, we observed that the numbers of collected mPB CD34+ cells showed a trend of correlation with the number of BM CD34+ cells at screening, as well as a significant correlation with CD34+ cell counts on the 5th day of mobilization (Figure S8).
Figure 4.
Relationship between basal PB and BM parameters determined at visit 0 and values of mPB CD34+ cells 5 days after initiation of the mobilization protocol.
(A–F) the relationship between numbers of mPB CD34+ cells at day 5 of mobilization and age of the patients and also basal hematological (leukocyte counts, mean corpuscular values, hemoglobin levels) and BM (percentage and total CD34+ cells/μL) parameters determined on visit 0. The dotted line in last panel shows the efficient HSPC mobilization (≥10 CD34+ cells/μL PB) of every patient containing at least 30 CD34+ cells/μL BM at visit 0. Patients’ color IDs are the same as in Figure 1. ∗p<0.05, ∗∗p<0.01.
Discussion
Previous studies have shown that HSPC numbers in the BM of FA patients are markedly lower compared to aged-matched HDs.9,10,21 Consequently, serious difficulties have been encountered in CD34+ cell collection for gene therapy in this population.11, 12, 13,18 The combination of filgrastim and plerixafor is now frequently utilized in different gene therapy trials22,23 and has been shown to significantly improve HSPC mobilization in FA mouse models.24 Nonetheless, the evaluation of this HSPC mobilization regimen in FA patients has never been performed to date. In this trial we show for the first time the efficacy of these drugs to facilitate the collection of mPB HSPCs from FA patients and reveal the absence of SAEs related to this treatment.
In terms of efficacy, nine out of the eleven enrolled patients (81.8%) mobilized to the threshold level of 5 CD34+ cells/μL PB required to initiate apheresis and reached a median value of 21.8 CD34+ cells/μL at the peak of mobilization. Since flow cytometry parameters used to analyze FA CD34+ cells can vary among laboratories, we also determined the fold increase of CD34+ cells at the peak of mobilization with respect to basal levels. Whereas previous studies showed that in FA patients G-CSF increased this ratio by 3.6-fold,12 our data show that the administration of filgrastim and plerixafor markedly increases CD34+ cells to levels almost 20-fold higher than the baseline, representing a clear advance compared to CD34+ cell mobilization with G-CSF monotherapy.
As deduced from the analysis of the 22 apheresis procedures conducted in this trial, a median value of 4.86 × 106 CD34+ cells/kg was collected in 2 or 3 apheresis procedures. Four of the nine patients in whom CD34+ cells were collected fulfilled the objective of the trial to collect at least 4 million CD34+ cells/kg of projected weight at 5 years. Nevertheless, the median number of CD34+ cells after immunoselection significantly decreased to 1.1 × 106 CD34+ cells/kg. Although the median CD34+ cell recovery of 32.0% could be considered acceptable for FA patients, it was of particular concern that in three cases the recovery was lower than 15% (Figure 2). In a previous clinical trial, Kelly et al. showed that the median CD34+ cell recovery in FA BM harvests was 48% (range 19%–133%).13 However, more recently, Adair et al. have reported a very low CD34+ cell recovery of 3%. These authors then generated a lineage depletion procedure that resulted in CD34+ cell recoveries of 70.7% and 94.6% in mPB and BM from HDs, with an overall reduction of 10-fold in the total number of nucleated cells.18 Because this procedure would imply the use of very high amounts of viral vectors for gene therapy purposes, in four immunoselections we decided only to modify the washing step during the CliniMACS immunoselection process. This simple modification allowed us to increase by 2.9-fold the recovery of CD34+ cells at the expense of reducing the purity of the CD34+ population by 6.6-fold. Although this procedure also implies the use of larger amounts of lentiviral vectors for gene therapy protocols, the low weight of these pediatric patients and the limited number of CD34+ cells generally collected from FA patients imply that costs associated with this modified procedure will not be very significant. Additionally, the modified immunoselection process results in the co-infusion of higher numbers of accessory cells, which might enhance the engraftment of transduced HSCs, as already shown in experimental models.25,26
Despite the obvious limitations associated with this trial due to the moderate number of enrolled patients and the fact that all of them belonged to the FA-A complementation group and that only one of them was female, we observed a number of relevant parameters with predictive value to identify FA patients with significant numbers of CD34+ cells to be used in gene therapy. In this respect, as shown in Figure 4, both patient age and several hematologic and hematopoietic parameters appear potentially predictive of the mobilization of CD34+ cells to PB.
Although CD34+ cell numbers represent a universal parameter to characterize hematopoietic content in HSC transplants and gene therapy programs, CD34+ cells constitute a heterogeneous population, whose content in primitive HSCs might be significantly reduced in stem cell diseases such as FA. As was shown in cells from one FA patient,18 and confirmed in the eleven patients of this trial, expression levels of the CD34 antigen in FA CD34+ cells were generally much lower compared to samples from HDs, suggesting a reduced HSC content in FA CD34+ cells.18,19
Consistent with this notion, previous experimental studies in transplanted NSG mice revealed that the repopulating potential of gene-corrected FA CD34+ cells was significantly lower compared to HD CD34+ cells.15 Based on precise HSC flow cytometry studies, we now demonstrate that numbers of primitive HSCs were 5-fold lower in FA compared to HD mPB CD34+ cells. Although the numbers of HSCs that could be collected from FA patients were 10–50 times lower compared to those collected for the gene therapy of other diseases,22,23,27 we have already demonstrated the ability of relatively low numbers of gene-corrected FA HSCs to engraft FA patients, even in the absence of any conditioning regimen.16 These results thus reinforce the remarkable repopulation potential and proliferation advantage of gene-corrected FA HSCs.
Taken together, the results obtained in this clinical trial show that HSPC mobilization with filgrastim and plerixafor constitutes a safe and efficient procedure that facilitates the collection of clinically relevant numbers of HSCs from pediatric FA patients. Additionally, our exploratory studies in a limited number of FA patients may help to identify FA patients capable of mobilizing significant HSPC quantities for being used in gene therapy protocols.
Materials and methods
FANCOSTEM-I (EudraCT: 2011-006197-88; ClinicalTrials.gov, NCT02931071; ECT 2011-006197-88) was a multicenter phase II clinical trial sponsored by Vall d’Hebrón Research Institut Foundation aiming at evaluating the safety and efficacy of filgrastim and plerixafor mobilization and subsequent collection of CD34+ cells in patients with FA for their subsequent use in gene therapy (the clinical protocol is included in Approved protocol). Briefly, the study consisted of a 5 day mobilization period that could be extended up to 8 days depending on the mobilization results and CD34+ cell collections. Up to four leukapheresis procedures were permitted to reach a target cell dose of 4 × 106 CD34+ cells/kg of weight estimated in a 5 year period (longest estimation time considered for the cryopreservation of mPB CD34+ cells in this trial). Patients treated in FANCOSTEM-I underwent a 1 year period of clinical and hematological follow-up (see details in Supplemental materials and methods). The clinical trial and subsequent amendments were approved by independent ethics committees at Hospital Universitari Vall d’Hebrón (Barcelona) and Hospital Infantil Universitario Niño Jesús (Madrid). All legal guardians of the patients provided written informed consent for the clinical trial, sample storage, and exploratory studies prior to enrollment.
Patients
Patients eligible for the study were patients under 18 years of age diagnosed with FA by chromosomal instability testing with DEB17 or MMC. All patients had confirmed mutational analysis.28 Patients were required to fulfill at least two of the following criteria: Hb ≥ 8 g/dL, neutrophils ≥ 0.75 × 109 cells/L, and platelets ≥ 30 × 109 cells/L. Additional details regarding inclusion and exclusion criteria are shown in the Supplemental materials and methods.
Treatment
Hospitalized patients were subcutaneously injected with filgrastim (Neupogen, twice daily; 12 μg/kg/12 h for up to 8 days). Plerixafor (Mozobil) was also administered subcutaneously at a dose of 240 μg/kg body weight/day (single dose) from the 4th and up to the 8th day after starting administration of filgrastim. At 3–5 h after each plerixafor administration, a PB sample was obtained for CD34+ cell analysis, and leukapheresis products and PB cells were analyzed locally for expression of CD34 antigen according to International Society of Hematotherapy and Graft Engineering (ISHAGE) guidelines.29 Details of CD34+ cell mobilization are described in the Supplementary materials and methods and are shown in Figure 1A. To evaluate the efficacy of filgrastim and plerixafor to mobilize HSPCs, numbers of PB CD34+ cells were determined daily from the first day of filgrastim treatment until the last apheresis procedure. CD34+/CD38− cells and CFCs were also analyzed and recorded during this time period.
Leukapheresis
Patients who achieved >5 CD34+ cells/μL underwent leukapheresis. Apheresis procedures were carried out via central venous catheter, which was placed after mild sedation or anesthesia. Collections were performed with a continuous flow blood cell separator (COBE Spectra TM, v.6.1, by Caridian BCT Europe, Garching, Germany; or more recently Spectra Optia+ MNC v.3.0, Terumo BCT, Lakewood, CO, USA). Acid citrate dextrose with a ratio of 12–14:1 was used as anticoagulant. Red blood cell priming of the blood cell separator was used for all patients under 15 kg or with mild anemia. Transfusion support was performed according to the clinical guidelines of Hospital Universitari Vall d’Hebron and Hospital Infantil Universitario Niño Jesús, and exposure to different donors was minimized as much as possible. Three to six patient blood volumes could be processed for every single collection (Table S1). Baseline pulse and blood pressure were measured and monitored during the leukapheresis process at regular intervals. A calcium gluconate infusion was used to prevent hypocalcemia if necessary. AEs related to the collection were recorded by the nursing staff of the apheresis units. At both institutions, this was a well-trained and experienced team for pediatric apheresis.
Immunoselection
Cells were collected daily and immediately processed for CD34+ cell immunoselection by the automated CliniMACS device (Miltenyi Biotec) following the manufacturer’s instructions. Minor modifications were included to improve CD34+ cell recovery of the final product. More details can be found in Supplemental materials and methods. The purity of CD34+ cells in the different steps of the collection/purification process was assessed by flow cytometry, as described in Supplemental materials and methods (Flow cytometry studies).
Safety
AEs were evaluated based on the Medical Dictionary for Regulatory Activities (MedDRA) terms and graded according to the interference in the patient’s quality of life (see Supplemental materials and methods for more details). AEs are presented in two groups. Events considered by the investigators as related to the mobilization agents (filgrastim and plerixafor) are classified as drug related. The other group is comprised of all AEs reported by patients and guardians for the complete trial period (catheter placement, HSPC collection, or follow-up period). AEs included in the former group were also part of the latter. During the mobilization treatment all patients and/or guardians were asked to report AEs, with special attention to headache, myalgia, low-grade fever, abdominal discomfort, bone pain, and vomiting.
Exploratory studies
Exploratory studies were performed for a post hoc analysis in order to evaluate pre-treatment variables that might be associated with either AEs or mobilization efficacy. Details of clonogenic assays, chromosomal breakage tests in PB T cells, and cytogenetic and fluorescence in situ hybridization (FISH) analyses were previously described16 and are detailed in Supplemental materials and methods.
Statistics
Patient and leukapheresis characteristics were expressed as the median and interquartile range (ICR) in boxes and range. D’Agostino and Pearson, Shapiro-Wilk, and Kolmogórov-Smirnov normality tests were applied for testing normality of the distribution in each cell population. According to non-Gaussian distributions unpaired non-parametric Mann-Whitney test was used to analyze statistical differences between the samples of multistem populations and the MFI of the different CD34+ populations. Wilcoxon signed-rank test was used to compare differences in mean values for paired variables (cells/mL; CD34+[%], CD34+/CD38− [%], and CFCs/105 cells) among the different apheresis and purified fractions. Continuous variables were represented according to semi-logarithmic regression. In order to find variables related to number of CD34+ cells/μL, Pearson correlation analyses was used for leukocytes, MCV, Hb, and CD34+ cells/kg, and Spearman correlation was used for age and proportion of CD34+ cells in BM aspiration at screening. Variables that were correlated in the univariate analysis were evaluated by stepwise linear regression analysis. All p values were two-sided, and p < 0.05 was considered significant.
Statistical analysis was performed with GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA) and SPSS version 22 (SPSS Inc., Chicago, IL, USA) software for Windows.
Acknowledgments
The authors would like to thank A. de la Cal for coordinating the delivery of BM and PB samples from patients with FA. The authors are also indebted to the patients with FA, their families, and clinicians from the Fundación Anemia de Fanconi. The authors also thank Lucie Hernández for processing samples for cytogenetic studies. This work was supported by grants from the European Commission’s Seventh Framework Program (HEALTH-F5-2012-305421 to the EUROFANCOLEN Consortium, J.A.B., J. Sevilla, C.D.-d.-H., J. Soulier, and J. Surralles), Ministerio de Sanidad, Servicios Sociales e Igualdad (EC11/060 and EC11/550 to C.D.-d.-H., J. Sevilla, J.A.B., and J. Surralles), Ministerio de Economía, Comercio y Competitividad and Fondo Europeo de Desarrollo Regional (SAF2015-68073-R, RTI2018-097125-B-I00 to P.R. and RTI2018-098419-B-I00 to J. Surralles), Fondo de Investigaciones Sanitarias at the Instituto de Salud Carlos III (RD12/0019/0023 to J.C.S.), and Consejería de Educación, Juventud y Deporte de la Comunidad de Madrid (AvanCell Project; B2017/BMD3692). CIBERER is an initiative of the Instituto de Salud Carlos III and Fondo Europeo de Desarrollo Regional. J. Surralles is supported by ICREA Academia and FARF.
Author contributions
J. Sevilla, S.N., P.R., J.A.B., and C.D.-d.-H. designed research. J. Sevilla, S.N., P.R., R.S.-D., J.Z., E.G., E.M., E.S., C.A., J.A.C., J.C.S., O.A., M.B., F.J.R.-R., Y.G., L.L., R.S., R.M.P., R.H., A. Castillo, J. Soulier, S.Q., J.F., N.G.d.A., R.L., A. Catalá, J. Surralles, C.D.-d.-H, and J.A.B. performed research. J. Sevilla, S.N., P.R., C.D.-d.-H, and J.A.B. analyzed data. J. Sevilla,∗ S.N.,∗ P.R.,∗ and J.A.B. wrote the paper, with J. Sevilla, S.N., and P.R. making equal contributions. All authors revised the paper, included comments, and accept the final version.
Declaration of interests
J. Sevilla is a consultant and advisor and has received honorarium (Amgen, Novartis, Miltenyi, Sobi, Rocket Pharmaceuticals Inc.) and has licensed medicinal products from Rocket Pharmaceuticals Inc. S.N. and P.R. have licensed medicinal products and receive research funding and equity from Rocket Pharmaceuticals Inc. J.C.S.: Rocket Pharmaceuticals Inc.: consultant/incomes from licensed medicinal products/research funding/equity. J. Schwartz is Medical Director of Rocket Pharmaceuticals Inc. J. Surralles: service agreements (Rocket Pharmaceuticals Inc.). J.A.B.: Rocket Pharmaceuticals Inc.: consultant/incomes from licensed medicinal products/research funding/equity; Roche: honorarium; Pfizer: honorarium.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2021.06.001.
Contributor Information
Julián Sevilla, Email: julian.sevilla@salud.madrid.org.
Juan A. Bueren, Email: juan.bueren@ciemat.es.
Supplemental information
References
- 1.Naldini L. Ex vivo gene transfer and correction for cell-based therapies. Nat. Rev. Genet. 2011;12:301–315. doi: 10.1038/nrg2985. [DOI] [PubMed] [Google Scholar]
- 2.Morgan R.A., Gray D., Lomova A., Kohn D.B. Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned. Cell Stem Cell. 2017;21:574–590. doi: 10.1016/j.stem.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tucci F., Frittoli M., Barzaghi F., Calbi V., Migliavacca M., Ferrua F., Fumagalli F., Lorioli L., Castagnaro L., Facchini M. Bone marrow harvesting from paediatric patients undergoing haematopoietic stem cell gene therapy. Bone Marrow Transplant. 2019;54:1995–2003. doi: 10.1038/s41409-019-0573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tisdale J.F., Pierciey F.J., Jr., Bonner M., Thompson A.A., Krishnamurti L., Mapara M.Y., Kwiatkowski J.L., Shestopalov I., Ribeil J.A., Huang W. Safety and feasibility of hematopoietic progenitor stem cell collection by mobilization with plerixafor followed by apheresis vs bone marrow harvest in patients with sickle cell disease in the multi-center HGB-206 trial. Am. J. Hematol. 2020;95:E239–E242. doi: 10.1002/ajh.25867. [DOI] [PubMed] [Google Scholar]
- 5.Wong J.C., Alon N., Norga K., Kruyt F.A., Youssoufian H., Buchwald M. Cloning and analysis of the mouse Fanconi anemia group A cDNA and an overlapping penta zinc finger cDNA. Genomics. 2000;67:273–283. doi: 10.1006/geno.2000.6252. [DOI] [PubMed] [Google Scholar]
- 6.Aubé M., Lafrance M., Brodeur I., Delisle M.C., Carreau M. Fanconi anemia genes are highly expressed in primitive CD34+ hematopoietic cells. BMC Blood Disord. 2003;3:1. doi: 10.1186/1471-2326-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceccaldi R., Sarangi P., D’Andrea A.D. The Fanconi anaemia pathway: new players and new functions. Nat. Rev. Mol. Cell Biol. 2016;17:337–349. doi: 10.1038/nrm.2016.48. [DOI] [PubMed] [Google Scholar]
- 8.Bagby G. Recent advances in understanding hematopoiesis in Fanconi Anemia. F1000Res. 2018;7:105. doi: 10.12688/f1000research.13213.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceccaldi R., Parmar K., Mouly E., Delord M., Kim J.M., Regairaz M., Pla M., Vasquez N., Zhang Q.S., Pondarre C. Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell. 2012;11:36–49. doi: 10.1016/j.stem.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larghero J., Marolleau J.P., Soulier J., Filion A., Rocha V., Benbunan M., Gluckman E. Hematopoietic progenitor cel harvest and functionality in Fanconi anemia patients. Blood. 2002;100:3051. doi: 10.1182/blood-2002-07-2069. [DOI] [PubMed] [Google Scholar]
- 11.Liu J.M., Kim S., Read E.J., Futaki M., Dokal I., Carter C.S., Leitman S.F., Pensiero M., Young N.S., Walsh C.E. Engraftment of hematopoietic progenitor cells transduced with the Fanconi anemia group C gene (FANCC) Hum. Gene Ther. 1999;10:2337–2346. doi: 10.1089/10430349950016988. [DOI] [PubMed] [Google Scholar]
- 12.Croop J.M., Cooper R., Fernandez C., Graves V., Kreissman S., Hanenberg H., Smith F.O., Williams D.A. Mobilization and collection of peripheral blood CD34+ cells from patients with Fanconi anemia. Blood. 2001;98:2917–2921. doi: 10.1182/blood.v98.10.2917. [DOI] [PubMed] [Google Scholar]
- 13.Kelly P.F., Radtke S., von Kalle C., Balcik B., Bohn K., Mueller R., Schuesler T., Haren M., Reeves L., Cancelas J.A. Stem cell collection and gene transfer in Fanconi anemia. Mol. Ther. 2007;15:211–219. doi: 10.1038/sj.mt.6300033. [DOI] [PubMed] [Google Scholar]
- 14.To L.B., Levesque J.P., Herbert K.E. How I treat patients who mobilize hematopoietic stem cells poorly. Blood. 2011;118:4530–4540. doi: 10.1182/blood-2011-06-318220. [DOI] [PubMed] [Google Scholar]
- 15.Río P., Navarro S., Guenechea G., Sánchez-Domínguez R., Lamana M.L., Yañez R., Casado J.A., Mehta P.A., Pujol M.R., Surrallés J. Engraftment and in vivo proliferation advantage of gene-corrected mobilized CD34+ cells from Fanconi anemia patients. Blood. 2017;130:1535–1542. doi: 10.1182/blood-2017-03-774174. [DOI] [PubMed] [Google Scholar]
- 16.Río P., Navarro S., Wang W., Sánchez-Domínguez R., Pujol R.M., Segovia J.C., Bogliolo M., Merino E., Wu N., Salgado R. Successful engraftment of gene-corrected hematopoietic stem cells in non-conditioned patients with Fanconi anemia. Nat. Med. 2019;25:1396–1401. doi: 10.1038/s41591-019-0550-z. [DOI] [PubMed] [Google Scholar]
- 17.Castella M., Pujol R., Callén E., Ramírez M.J., Casado J.A., Talavera M., Ferro T., Muñoz A., Sevilla J., Madero L. Chromosome fragility in patients with Fanconi anaemia: diagnostic implications and clinical impact. J. Med. Genet. 2011;48:242–250. doi: 10.1136/jmg.2010.084210. [DOI] [PubMed] [Google Scholar]
- 18.Adair J.E., Chandrasekaran D., Sghia-Hughes G., Haworth K.G., Woolfrey A.E., Burroughs L.M., Choi G.Y., Becker P.S., Kiem H.P. Novel lineage depletion preserves autologous blood stem cells for gene therapy of Fanconi anemia complementation group A. Haematologica. 2018;103:1806–1814. doi: 10.3324/haematol.2018.194571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baech J., Johnsen H.E. Technical aspects and clinical impact of hematopoietic progenitor subset quantification. Stem Cells. 2000;18:76–86. doi: 10.1634/stemcells.18-2-76. [DOI] [PubMed] [Google Scholar]
- 20.Morland B., Kepak T., Dallorso S., Sevilla J., Murphy D., Luksch R., Yaniv I., Bader P., Rößler J., Bisogno G. Plerixafor combined with standard regimens for hematopoietic stem cell mobilization in pediatric patients with solid tumors eligible for autologous transplants: two-arm phase I/II study (MOZAIC) Bone Marrow Transplant. 2020;55:1744–1753. doi: 10.1038/s41409-020-0836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacome A., Navarro S., Río P., Yañez R.M., González-Murillo A., Lozano M.L., Lamana M.L., Sevilla J., Olive T., Diaz-Heredia C. Lentiviral-mediated genetic correction of hematopoietic and mesenchymal progenitor cells from Fanconi anemia patients. Mol. Ther. 2009;17:1083–1092. doi: 10.1038/mt.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marktel S., Scaramuzza S., Cicalese M.P., Giglio F., Galimberti S., Lidonnici M.R., Calbi V., Assanelli A., Bernardo M.E., Rossi C. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent ß-thalassemia. Nat. Med. 2019;25:234–241. doi: 10.1038/s41591-018-0301-6. [DOI] [PubMed] [Google Scholar]
- 23.Thompson A.A., Walters M.C., Kwiatkowski J., Rasko J.E.J., Ribeil J.A., Hongeng S., Magrin E., Schiller G.J., Payen E., Semeraro M. Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia. N. Engl. J. Med. 2018;378:1479–1493. doi: 10.1056/NEJMoa1705342. [DOI] [PubMed] [Google Scholar]
- 24.Pulliam A.C., Hobson M.J., Ciccone S.L., Li Y., Chen S., Srour E.F., Yang F.C., Broxmeyer H.E., Clapp D.W. AMD3100 synergizes with G-CSF to mobilize repopulating stem cells in Fanconi anemia knockout mice. Exp. Hematol. 2008;36:1084–1090. doi: 10.1016/j.exphem.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Loo J.C., Hanenberg H., Cooper R.J., Luo F.Y., Lazaridis E.N., Williams D.A. Nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse as a model system to study the engraftment and mobilization of human peripheral blood stem cells. Blood. 1998;92:2556–2570. [PubMed] [Google Scholar]
- 26.Fernández-García M., Luisa Lamana M., Hernando-Rodríguez M., Sánchez-Domínguez R., Bueren J., Yañez R. Improved Hematopoietic Gene Therapy in a Mouse Model of Fanconi Anemia Mediated by Mesenchymal Stromal Cells. Hum. Gene Ther. 2018;29:327–336. doi: 10.1089/hum.2017.076. [DOI] [PubMed] [Google Scholar]
- 27.Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., Veres G., Schmidt M., Kutschera I., Vidaud M., Abel U., Dal-Cortivo L., Caccavelli L. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 28.Bogliolo M., Pujol R., Aza-Carmona M., Muñoz-Subirana N., Rodriguez-Santiago B., Casado J.A., Rio P., Bauser C., Reina-Castillón J., Lopez-Sanchez M. Optimised molecular genetic diagnostics of Fanconi anaemia by whole exome sequencing and functional studies. J. Med. Genet. 2020;57:258–268. doi: 10.1136/jmedgenet-2019-106249. [DOI] [PubMed] [Google Scholar]
- 29.Keeney M., Chin-Yee I., Weir K., Popma J., Nayar R., Sutherland D.R., International Society of Hematotherapy and Graft Engineering Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. Cytometry. 1998;34:61–70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.