Abstract
Lethality and cytotoxicity assays of snake venoms and their neutralization by antivenom require many mice for the experiments. Recent developments have prompted researchers to seek alternative strategies that minimize the use of mice in line with Russel and Burch's 3Rs philosophy (Replacement, Reduction, and Refinement). Artemia salina is an animal model widely used for toxicity screening. However, its use in snake venom toxinology is limited by a lack of data. The present study compared the toxicity of venoms from Bitis arietans, Naja ashei, and Naja subfulva using mice and Artemia salina. In the Artemia salina test at 24 h and the dermonecrotic test in mice, the toxicity of the venoms was in the order Naja ashei ~ Naja subfulva > Bitis arietans. In the lethality test in mice, the toxicity of the venoms was in the order Naja subfulva > Naja ashei > Bitis arietans. These findings suggest that the toxicity of the venoms in Artemia salina and the dermonecrotic bioassay in mice have a similar trend but differ from the lethality test in mice. Therefore, it may be relevant to further explore the Artemia salina bioassay as a potential surrogate test of dermonecrosis in mice. Studies with more venoms may be needed to establish the correlation between the Artemia salina bioassay and the dermonecrotic assay in mice.
Keywords: Artemia salina, Mice, Lethal concentration, Lethal dose, Necrosis
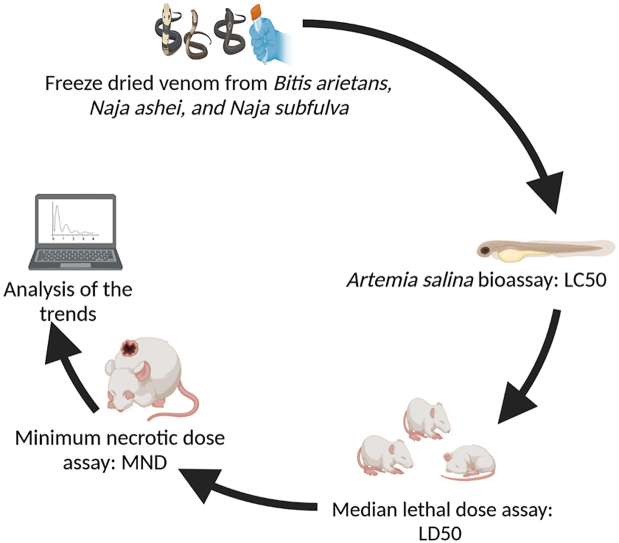
Graphical abstract
Highlights
-
•
Bitis arietans, Naja ashei, and Naja subfulva snake venom-induced toxicities were compared in Artemia salina and in mice.
-
•
Venom-induced toxicity in Artemia salina has a similar trend to necrosis in mice but not lethality.
-
•
The Artemia salina bioassay may be further explored along the 3Rs (Reduction, Replacement, and Refinement) concept.
1. Introduction
Antivenom efficacy is primarily evaluated in mice using the neutralization of lethality assay (WHO, 2016). This is a preclinical test that is routinely carried out by antivenom manufacturers in fulfillment of regulatory requirements (Gutiérrez et al, 2013, 2021). Investigating the capacity of antivenoms to neutralize other toxic effects of venom e.g. dermonecrosis may also be important (Gutiérrez et al., 2021; 2013; WHO, 2016). Towards this end, the World Health Organization (WHO) recommends the in vivo minimum necrotizing dose (MND) assay in mice (WHO, 2016).
Assays on the toxicities of venom e.g. lethality and dermonecrosis and their neutralization by antivenom require many mice for the experiments, which are costly and labor-intensive. Criticism by animal welfare groups is also rife (da Silva et al., 2015; Kakanj et al., 2015; Kerkkamp et al., 2018; Stransky et al., 2018). Therefore, researchers are motivated to seek alternative techniques which may significantly minimize the use of or eliminate the need to use mice (Calvete et al., 2016; Barbosa et al., 1995; Chacón et al., 2015; De souza et al., 2015; Khaing et al., 2018; Oguiura et al., 2014; Pornmuttakun and Ratanabanangkoon, 2014; Rial et al., 2006; Rungsiwongse and Ratanabanangkoon, 1991; Segura et al., 2010; Theakston and Reid, 1979).
The lethality assay in brine shrimp (Artemia salina) is rapid and requires minimal resources (Hamidi et al., 2014). It has many applications in toxicology (Barahona and Sanchez-Fortun, 1999; Gadir, 2012; Hernández-Matehuala et al., 2015; Kerster and Schaeffer, 1983; Mirzaei and Mirzaei, 2013; Mwangi et al., 2015; Okumu et al., 2020; Hamidi et al., 2014; Rajabi et al., 2015, 2012). However, its suitability as a replacement or surrogate test to the more technically and ethically challenging lethality or necrotizing assays is limited by a lack of data. This study compared the Artemia salina lethality assay with the lethality and necrotizing assays in mice using venom from some snakes of medical importance in Sub-Saharan Africa namely Bitis arietans (Puff adder), Naja ashei (large brown spitting cobra), and Naja subfulva (Eastern Forest Cobra).
2. Materials and methods
2.1. Ethical considerations
Ethical approval was obtained from the institutional Biosafety, Animal Care, and Use Committee REF BAUEC/2019/220.
2.2. Snake venom
Venom was collected from snakes (Bitis arietans, Naja ashei, and Naja subfulva) which are maintained in Kenya (Bioken Snake Farm, Malindi). Freshly collected venom from each snake species was pooled, snap frozen, lyophilized, and stored at −20 °C. Freeze-dried venom samples were reconstituted in phosphate-buffered saline at the time of use.
2.3. Artemia salina (brine shrimp) lethality assay
Artemia salina (brine shrimp) eggs were commercially sourced (Batch number; X001M8M5IZ) and hatched in a trough using marine salt solution (MSS; 38.5% w/v) over 48 h. Artemia nauplii/larvae were transferred from the hatching trough to 5 mL sample vials. Venom aliquots (5, 50, and 500 μL) were pipetted from venom stock solutions (5 mg/mL) into the vials which contained brine shrimp. The contents were made up to the mark with MSS resulting in 10, 100, and 1000 μg/mL venom concentrations (Meyer et al., 1982). The nauplii that died were counted after 24 and 72 h. Experiments were carried out in quintuples of 10 nauplii per vial. Mortality was analyzed by probit analysis and the results were expressed as LC50: Median lethal concentration (Bliss, 1935; Finney, 1952). In cases where there were deaths in the control group (phosphate-buffered saline), the data were corrected using the formula by Abbot (Abbott, 1925).
2.4. The median lethal dose (LD50) assay in mice
Eighty mice weighing 18–20 g were randomly assigned to 16 groups of 5 mice each. Graded doses of Bitis arietans, Naja ashei, and Naja subfulva venoms were prepared in phosphate-buffered saline and administered to 75 mice (WHO, 2016). Five mice served as control and received phosphate-buffered saline only. Mortality was recorded after 24 h and the least dose of Bitis arietans, Naja ashei, and Naja subfulva venom which was responsible for 50% mortality was determined by Probit analysis (Finney, 1952).
2.5. The minimum necrotizing dose (MND) assay in mice
Eighty mice weighing 18–20 g were shaved (Theakston and Reid, 1983; WHO, 2016). Solutions (50 μL) containing graded doses of Bitis arietans, Naja ashei, and Naja subfulva venoms were injected intradermally in the shaved skin of mice (Theakston and Reid, 1983; WHO, 2016). After 3 days, the mice were humanely sacrificed and the skin was carefully removed. The diameter of snake venom-induced necrotic lesions on the inner side of the skin was measured using a digital Vernier caliper (Theakston and Reid, 1983; WHO, 2016). A plot of the mean lesion diameter against the venom doses was used to estimate the minimum necrotizing dose (MND) which was defined as the dose of venom which corresponded to a 5-mm necrotic lesion (Theakston and Reid, 1983; WHO, 2016). A negative control group received phosphate-buffered saline only.
2.6. Statistical analysis
The Artemia salina LC50 values of the venoms were determined by probit analysis (Bliss, 1935; Gaddum, 1948). The minimum necrotizing dose was defined as the least amount of venom which when injected intradermally produced a necrotic lesion of 5 mm diameter. It was estimated by plotting mean lesion diameter against venom dose and reading off the dose which corresponded to a 5-mm diameter (Theakston and Reid, 1983; WHO, 2016).
3. Results
Information on the gender, size, location of capture, and reference number of the snakes used in venom extraction is shown in Table S1. Adult, male and female snakes were collected from Watamu, Kizingo, the Arabuko Sokoke Forest, Kilifi, Kakamega, Busia, and Nandi. Table S1.
The mortalities of Artemia salina larvae treated with phosphate-buffered saline only (negative control) are summarized in Table S2. Some mortality was observed in controls at 72 h. Bitis arietans, Naja ashei, and Naja subfulva snake venom-induced mortality in Artemia salina is summarized in Tables S3, S4, and S5 respectively. Generally, there were dose and time-dependent increases in the venom-induced mortalities of Artemia salina. See Tables S3, S4, and S5.
According to the data of Table 1, the toxicity of snake venoms in Artemia salina after 24 h was in the order Naja ashei ~ Naja subfulva > Bitis arietans. Table 1. The toxicity (lethality) of the venoms in mice after 24 h was in the order Naja subfulva > Naja ashei > Bitis arietans. Table 1. The necrotizing activity of the venoms in mice after 72 h of exposure was in the order Naja subfulva ~ Naja ashei > Bitis arietans. Table 1.
Table 1.
Comparison of the toxicity of Bitis arietans, Naja ashei and Naja subfulva snake venoms in Artemia salina and in mice.
| Toxicity in Artemia salinaa | Toxicity in mice | |||
|---|---|---|---|---|
| Snake species | LC50 (24 h) | LC50 (72 h) | LD50 (24 h)b | MND (72 h)c |
| Bitis arietans | 410.47 (228.21–729.14) | 57.36 (26.50–125.47) | 9.24 (7.50–11.38) | 112.92 |
| Naja ashei | 41.28 | 5.40 | 3.02 (2.45–3.72) | 36.12 |
| Naja subfulva | 47.54 | 12.06 | 0.55 (0.49–0.63) | 31.48 |
Expressed as Median Lethal Concentration (LC50) in μg/mL, with 95% confidence limits in parentheses.
Expressed as Median Lethal Dose (LD50) in μg/g, with 95% confidence limits in parentheses.
Expressed as Minimum Necrotizing Dose (MND) in μg.
4. Discussion
The search for alternatives to the mouse lethality/toxicity assays in snake venom research has been a subject of fascination for many researchers past and present. As far back as 1907, Albert Calmette described a relationship between toxicity in mice and in vitro proteolysis and hemotoxicity (Calmette, 1907). Russel and Burch's 3Rs philosophy (Reduction, Replacement, and Refinement) limits the number of mice used in experimental assays and is widely celebrated in the snake venom toxinology community (Russel and Burch 1959). In line with this philosophy, the present study presents baseline data on the applicability of an alternative animal model for the preliminary evaluation of snake venom-induced toxicity.
Lethality is an acute response to venom while dermonecrosis takes some time to unravel (i.e. up to 72 h) (WHO, 2016). In the present study, murine LD50 and Artemia salina bioassays were compared at different times i.e. the toxicity in Artemia salina (24 and 72 h) and the toxicity in mice (24 and 72 h). A good relationship was observed between the 24 h Artemia salina bioassay and the 72-h dermonecrosis bioassay. In other words, the Artemia salina test does not necessarily have to be done at 72 h. However, a major problem of running the Artemia salina test for an extended period (72 h) was the high number of deaths in the negative control (phosphate-buffered saline) samples. Therefore, based on these results, the best time to judge the lethality of Artemia salina would be after 24 h.
The mortality trends of the venom in Artemia salina were inconsistent with mortality in mice. On the strength of these findings, the Artemia salina model does not appear to be suitable for evaluating neurotoxic venoms. It could be argued that the neurotoxins of elapid venom (Naja subfulva), most of which act on nicotinic acetylcholine receptors of muscle cells (Kukhtina et al., 2000), do not act on the synapsis of Artemia salina. However, the observation that high doses of the venoms were able to kill the Artemia salina suggests that there may be some neurotoxic effect realized. Perhaps these neurotoxins cannot readily penetrate the tissues of Artemia salina.
It was interesting to note that the cytotoxic venom (Naja ashei) was more toxic to Artemia salina at 72 h than the neurotoxic Naja subfulva venom. This could imply that the cytotoxins (either 3FTxs or PLA2s) in Naja ashei venom (Hus et al., 2018) have the capacity to damage membranes of the Artemia salina. The findings of this study when taken together with the advantages of the Artemia salina assay (affordability, rapidity, simplicity, convenience, and robustness) and its utility in antivenom efficacy testing (Okumu et al., 2020) make a strong case for its’ use as an alternative to the more technically challenging, and expensive minimum necrotizing dose assay in mice. However, despite the many positive aspects of the assay, there are some drawbacks associated with the use of Artemia salina. First, it does not provide adequate information on the mechanism of cytotoxicity of the test substance (Hamidi et al., 2014). Secondly, the hatching of the Artemia nauplii requires a container that should be large enough to hold an air pump for equal distribution of oxygen (Vanhaecke et al., 1981). Moreover, 48-h old nauplii (2nd to 3rd instar stage) are more sensitive to test compounds (Vanhaecke et al., 1981). Thus many of the nauplii which hatch beyond the 48-h incubation period may not be suitable for experimental assays. Variations in pH and temperature may also affect the hatching process of the nauplii (Sorgeloos et al., 1978).
5. Conclusion
These findings suggest that the toxicity of the venoms in Artemia salina and the dermonecrotic bioassay in mice have a similar trend. Therefore, it may be relevant to further explore the Artemia salina bioassay along the lines of the 3Rs concept aimed at reducing the use of mice in snake venom research. Studies with a larger number of venoms are warranted to determine the correlation between the Artemia salina bioassay and the dermonecrotic assay in mice.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to acknowledge Ms. Clare Taylor and Boniface Momanyi of Bioken snake farm (Watamu, Kenya) for providing the venom samples. We would also like to thank Mr. Joseph Nderitu and Mr. Kenneth Maloba of the Department of Public Health, Pharmacology and Toxicology, the University of Nairobi for their assistance in conducting some of the assays. The graphical abstract was designed using Biorender.
Handling Editor: Dr. Raymond Norton
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2021.100082.
Contributor Information
Mitchel Otieno Okumu, Email: drokumitch@students.uonbi.ac.ke.
James Mucunu Mbaria, Email: james.mbaria@uonbi.ac.ke.
Joseph Kangangi Gikunju, Email: jgikunju@jkuat.ac.ke.
Paul Gichohi Mbuthia, Email: pgmbuthia@uonbi.ac.ke.
Vincent Odongo Madadi, Email: vmadadi@uonbi.ac.ke.
Francis Okumu Ochola, Email: ochola.francis@yahoo.com.
Mercy Seroney Jepkorir, Email: seroneymercy@yahoo.com.
Author contributions
Conceptualization: MO, FO, and JG; data curation: all authors; formal analysis: MO; investigation: all authors; methodology: MO, FO, and MJ.; project administration: MO, JG, and FO, resources; all authors; software: MO; supervision; JM, JG, PM, and VM; validation: FO, JM, JG, PM, VM, and MJ; visualization: MO, and MJ; writing original draft: MO and FO: writing review and editing: all authors. All authors read and approved the final manuscript.
Ethical considerations
This study was approved by the Biosafety, Animal Care, and Use Committee of the Faculty of Veterinary Medicine, University of Nairobi. REF BAUEC/2019/220 which was issued on April 24, 2019. All the used cell lines were disposed of per the Kenya Medical Research Institute (KEMRI)-University of Nairobi (UoN) protocols.
Consent for publication
Not applicable.
Funding
This study was funded by the National Research Fund (REF NRF/Ph.D./02/158).
Abbreviations
MND Minimum necrotizing dose; WHO: World Health Organization; BAUEC: Biosafety Animal Use and Ethics Committee; KEMRI: Kenya Medical Research Institute; UoN: University of Nairobi; LC50: the lethal concentration responsible for 50% mortality in Artemia salina; LD50; the median lethal dose responsible for 50% mortality in mice.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. [Google Scholar]
- Barahona M.V., Sanchez-Fortun S. Toxicity of carbamates to the brine shrimp Artemia salina and the effect of atropine, BW284c51, iso-OMPA, and 2-PAM on carbaryl toxicity. Environ. Pollut. 1999;104:469–476. [Google Scholar]
- Barbosa C.F., Rodrigues R.J., Olortegui C.C., Sanchez E.F., Heneine L.G. Determination of the neutralizing potency of horse antivenom against bothropic and crotalic venoms by indirect enzyme immunoassay. Brazilian Journal of Medical and Biological Research. Revista Brasileira de Pesquisas Medicas e Biologicas. 1995;28:1077–1080. [PubMed] [Google Scholar]
- Bliss C.I. The calculation of the dosage‐mortality curve. Ann. Appl. Biol. 1935;22:134–167. doi: 10.1111/j.1744-7348.1935.tb07713.x. [DOI] [Google Scholar]
- Calmette A. Masson; 1907. Les venins: les animaux venimeux et la serothorapie antivemeuse. [Google Scholar]
- Calvete J.J., Arias A.S., Rodríguez Y., Quesada-Bernat S., Sánchez L.V., Chippaux J.P., Pla D., Gutiérrez J.M. Preclinical evaluation of three polyspecific antivenoms against the venom of Echis ocellatus: neutralization of toxic activities and antivenomics. Toxicon. 2016;119:280–288. doi: 10.1016/J.TOXICON.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Chacón F., Oviedo A., Escalante T., Solano G., Rucavado A., Gutiérrez J.M. The lethality test used for estimating the potency of antivenoms against Bothrops asper snake venom: pathophysiological mechanisms, prophylactic analgesia, and a surrogate in vitro assay. Toxicon. 2015;93:41–50. doi: 10.1016/J.TOXICON.2014.11.223. [DOI] [PubMed] [Google Scholar]
- da Silva C.P., Costa T.R., Paiva R.M.A., Cintra A.C.O., Menaldo D.L., Antunes L.M.G., Sampaio S.V. Antitumor potential of the myotoxin BthTX-I from Bothrops jararacussu snake venom: evaluation of cell cycle alterations and death mechanisms induced in tumor cell lines. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015;21:1–8. doi: 10.1186/s40409-015-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De souza L.L., Stransky S., Guerra-duarte C., Flor-sá A., Schneider F.S., Kalapothakis E., Chávez-olórtegui C. Determination of toxic activities in Bothrops spp. snake venoms using animal-free approaches: correlation between in vitro versus in vivo assays. Toxicol. Sci. 2015;147:458–465. doi: 10.1093/toxsci/kfv140. [DOI] [PubMed] [Google Scholar]
- Finney D.J. Cambridge university press; Cambridge: 1952. Probit Analysis: Statistical Treatment of the Sigmoid Response Curve. [Google Scholar]
- Gaddum J.H. Probit analysis. Nature. 1948;161:417–418. doi: 10.1038/161417a0. [DOI] [Google Scholar]
- Gadir S.A. Assessment of bioactivity of some Sudanese medicinal plants using brine shrimp (Artemia salina) lethality assay. J. Chem. Pharmaceut. Res. 2012;4:5145–5148. [Google Scholar]
- Gutiérrez J.M., Solano G., Pla D., Herrera M., Segura Á., Villalta M., Vargas M., Sanz L., Lomonte B., Calvete J.J., León G. Assessing the preclinical efficacy of antivenoms: from the lethality neutralization assay to antivenomics. Toxicon. 2013;69:168–179. doi: 10.1016/J.TOXICON.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Vargas M., Segura A., Herrera M., Villalta M., Solano G., Sánchez A., Herrera C., León G. In vitro tests for assessing the neutralizing ability of snake antivenoms: toward the 3Rs principles. Front. Immunol. 2021:3394. doi: 10.3389/FIMMU.2020.617429. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M., Jovanova B., Kadifkova Panovska T. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Macedonian Pharmaceutical Bulletin. 2014;60:9–18. doi: 10.33320/maced.pharm.bull.2014.60.01.002. [DOI] [Google Scholar]
- Hernández-Matehuala R., Rojas-Molina A., Vuelvas-Solórzano A.A., Garcia-Arredondo A., Alvarado C.I., Olguín-López N., Aguilar M. Cytolytic and systemic toxic effects induced by the aqueous extract of the fire coral Millepora alcicornis collected in the Mexican Caribbean and detection of two types of cytolisins. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015;21:1–13. doi: 10.1186/s40409-015-0035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus K.K., Buczkowicz J., Petrilla V., Petrillová M., Łyskowski A., Legáth J., Bocian A. First look at the venom of Naja ashei. Molecules. 2018;23:609. doi: 10.3390/molecules23030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakanj M., Ghazi-Khansari M., Mirakabadi A.Z., Daraei B., Vatanpour H. Cytotoxic effect of Iranian Vipera lebetina snake venom on HUVEC cells. Iran. J. Pharm. Res. (IJPR): IJPR. 2015;14:109. [PMC free article] [PubMed] [Google Scholar]
- Kerkkamp H., Bagowski C., Kool J., Soolingen B. Van, Vonk F.J., Vlecken D. Toxicon Whole snake venoms: cytotoxic, anti-metastatic and antiangiogenic properties. Toxicon. 2018;150:39–49. doi: 10.1016/j.toxicon.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Kerster H.W., Schaeffer D.J. Brine shrimp (Artemia salina) nauplii as a teratogen test system. Ecotoxicol. Environ. Saf. 1983;7:342–349. doi: 10.1016/0147-6513(83)90079-9. [DOI] [PubMed] [Google Scholar]
- Khaing E.M., Hurtado P.R., Hurtado E., Zaw A., White J., Warrell D.A., Alfred S., Mahmood M.A., Peh C.A. Development of an ELISA assay to determine the neutralising capacity of horse serum following immunization with Daboia siamensis venom in Myanmar. Toxicon. 2018;151:163–168. doi: 10.1016/J.TOXICON.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Kukhtina V.V., Weise C., Muranova T.A., Starkov V.G., Franke P., Hucho F., Wnendt S., Gillen C., Tsetlin V.I., Utkin Y.N. Muscarinic toxin-like proteins from cobra venom. Eur. J. Biochem. 2000;267:6784–6789. doi: 10.1046/j.1432-1327.2000.01775.x. [DOI] [PubMed] [Google Scholar]
- Meyer B.N., Ferrigni N.R., Putnam J.E., Jacobsen L.B., Nichols D.E. j, McLaughlin J.L. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. [PubMed] [Google Scholar]
- Mirzaei M., Mirzaei A. Comparison of the Artemia salina and Artemia uramiana bioassays for toxicity of 4 Iranian medicinal plants. Int. Res. J. Biol. Sci. 2013;2:49–54. [Google Scholar]
- Mwangi G.G., Wagacha J.M., Nguta J.M., Mbaria J.M. Brine shrimp cytotoxicity and antimalarial activity of plants traditionally used in the treatment of malaria in Msambweni district. Pharmaceut. Biol. 2015;53:588–593. doi: 10.3109/13880209.2014.935861. [DOI] [PubMed] [Google Scholar]
- Oguiura N., Kapronezai J., Ribeiro T., Rocha M.M.T., Medeiros C.R., Marcelino J.R., Prezoto B.C. An alternative micro method to access the procoagulant activity of Bothrops jararaca venom and the efficacy of antivenom. Toxicon. 2014;90:148–154. doi: 10.1016/J.TOXICON.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Okumu M.O., Mbaria J.M., Gikunju J.K., Mbuthia P.G., Madadi V.O., Ochola F.O. Enzymatic activity and brine shrimp lethality of venom from the large brown spitting cobra (Naja ashei) and its neutralization by antivenom. BMC Res. Notes. 2020;13:1–7. doi: 10.1186/s13104-020-05167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornmuttakun D., Ratanabanangkoon K. Development of an in vitro potency assay for antivenom against Malayan pit viper (Calloselasma rhodostoma) Toxicon. 2014;77:1–5. doi: 10.1016/J.TOXICON.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Rajabi S., Ramazani A., Hamidi M., Naji T. Biochemical tolerance during low dose propylene glycol exposure in neonates: a formulation-controlled evaluation. 2012. [DOI] [PMC free article] [PubMed]
- Rajabi S., Ramazani A., Hamidi M., Naji T. Artemia salina as a model organism in toxicity assessment of nanoparticles 1–6. 2015. [DOI] [PMC free article] [PubMed]
- Rial A., Morais V., Rossi S., Massaldi H. A new ELISA for determination of potency in snake antivenoms. Toxicon. 2006;48:462–466. doi: 10.1016/J.TOXICON.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rungsiwongse J., Ratanabanangkoon K. Development of an ELISA to assess the potency of horse therapeutic antivenom against Thai cobra venom. J. Immunol. Methods. 1991;136:37–43. doi: 10.1016/0022-1759(91)90247-D. [DOI] [PubMed] [Google Scholar]
- Russel W.M.S., Burch R.L. Methuen & Co; London: 1959. The Principles of Humane Experimental Technique.http://117.239.25.194:7000/jspui/bitstream/123456789/1342/1/PRILIMINERY%20%20AND%20%20CONTENTS.pdf Available from: [Google Scholar]
- Segura A., Castillo M.C., Núñez V., Yarlequé A., Gonçalves L.R. de C., Villalta M., Bonilla C., Herrera M., Vargas M., Fernández M. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically relevant Bothrops snake venoms. Toxicon. 2010;56:980–989. doi: 10.1016/j.toxicon.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Sorgeloos P., Remiche-Van Der Wielen C., Persoone G. The use of Artemia nauplii for toxicity tests—a critical analysis. Ecotoxicol. Environ. Saf. 1978;2:249–255. doi: 10.1016/s0147-6513(78)80003-7. [DOI] [PubMed] [Google Scholar]
- Stransky S., Costal-Oliveira F., Lopes-de-Souza L., Guerra-Duarte C., Chávez-Olórtegui C., Braga V.M.M. In vitro assessment of cytotoxic activities of Lachesis muta muta snake venom. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theakston R.D.G., Reid H.A. Enzyme-linked Immunosorbent Assay (ELISA) in assessing antivenom potency. Toxicon. 1979;17:511–515. doi: 10.1016/0041-0101(79)90284-8. [DOI] [PubMed] [Google Scholar]
- Theakston R.D.G., Reid H.A. Development of simple standard assay procedures for the characterization of snake venoms. Bull. World Health Organ. 1983;61:949. [PMC free article] [PubMed] [Google Scholar]
- Vanhaecke P., Persoone G., Claus C., Sorgeloos P. Proposal for a short-term toxicity test with Artemia nauplii. Ecotoxicol. Environ. Saf. 1981;5:382–387. doi: 10.1016/0147-6513(81)90012-9. [DOI] [PubMed] [Google Scholar]
- WHO Post-ECBS version expert committee on biological standardization geneva, 17 to 21 october 2016. WHO guidelines for the production, control, and regulation of snake antivenom immunoglobulins. World Health Organization. 2016:1–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.