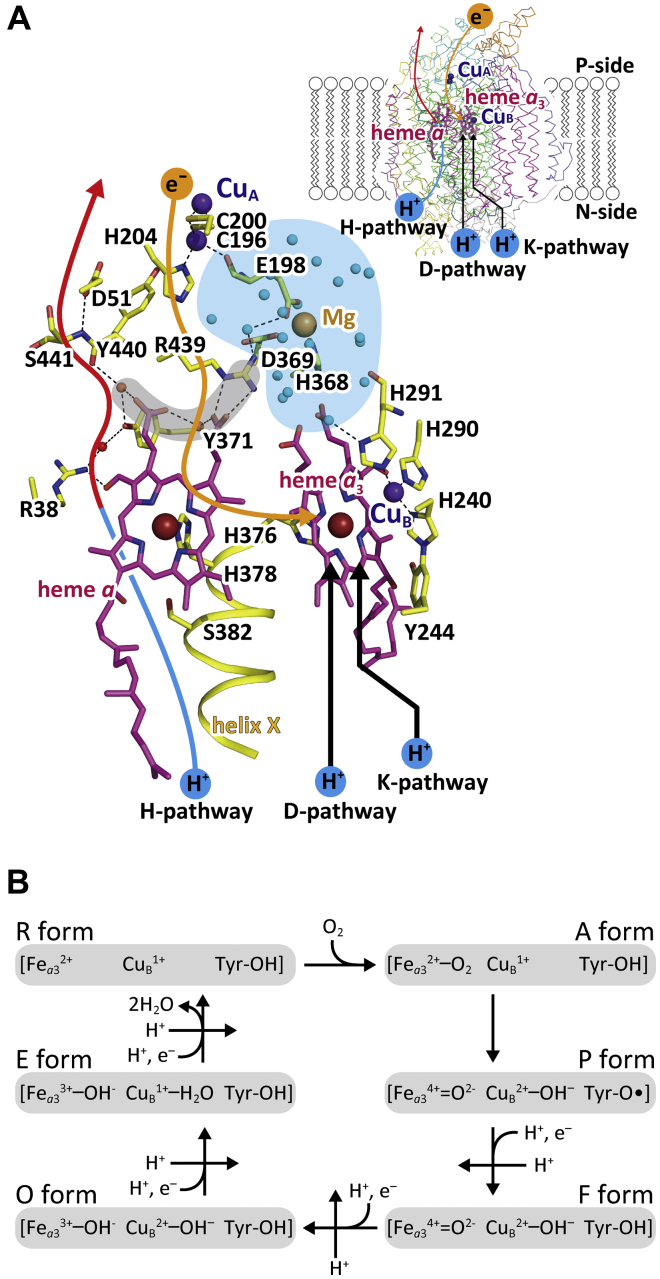
Figure 1.
X-ray structure of the active sites and a schematic representation of the catalytic cycle of bovine heart CcO.A, X-ray structure of the active sites. Metal sites are indicated by brown, violet, and beige spheres for iron, copper, and magnesium ions, respectively. Porphyrins of heme a and heme a3 are represented by the magenta stick models as labeled. Within the stick models of the amino acid residues, dark blue, red, and yellow portions are nitrogen, oxygen, and carbon, respectively. A beige arrow indicates the location of the electron transfer passage, while two black arrows indicate those for protons for producing water molecules. The hydrogen-bond network and the water channel of the H-pathway are indicated by the red and blue portions of the leftmost curved arrow, respectively. The Mg/H2O cluster (the blue area) is attached to the hydrogen-bond network of the H-pathway via a short hydrogen-bond network (the gray area). Small blue spheres in the Mg/H2O cluster mark the positions of water molecules, while small beige spheres mark the other water molecule positions. The formyl group and one of the propionate groups of heme a are hydrogen-bonded with Arg38 and a fixed water molecule in the hydrogen-bond network of the H-pathway. The inset shows the overall locations of the redox-active metal sites and pathways for transportation of electrons and protons within the CcO structure, indicated by Cα-backbone traces. This figure was prepared from the X-ray diffraction data of PDB 5B1A. B, a schematic representation of the structural changes in the O2 reduction site of CcO; Fea3 and CuB are the iron and copper ions; Tyr-OH and Tyr-O• denote Tyr244 located in its protonated and neutral radical states, respectively. The six intermediate forms in the catalytic cycle are designated as A-to R-forms. The reaction steps coupled with proton pumping are indicated by straight arrows marked with “H+”. This figure is a slightly modified version of the previous paper (23).