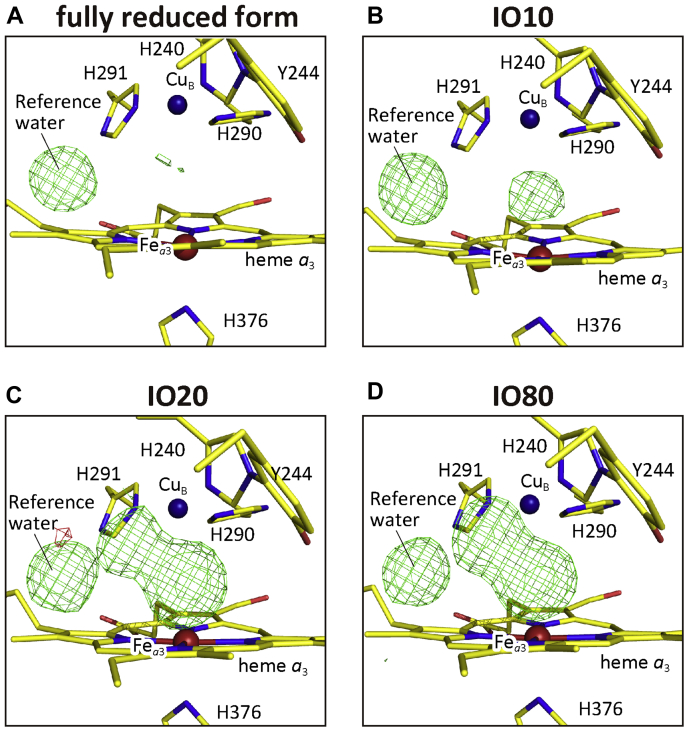
Figure 7.
Effect of O2 exposure periods on the electron density between Fea3 and CuB.Fo-Fc maps of the O2 reduction site of the X-ray structures of the re-refined fully reduced form (A), IO10 (B), IO20 (C), and IO80 (D) in monomer A at 1.80 Å resolution. Refinement was performed on a structure in the absence of a ligand in the O2-reduction site and without the water molecule bridging the two propionate groups of heme a3, and the Fo-Fc map was calculated. The electron density cages were drawn at 3.0 σ. Structures of proteins and hemes are drawn as stick models, and the Fe atoms in heme a3 and Cu atoms in CuB site are indicated by red and dark blue spheres, respectively.