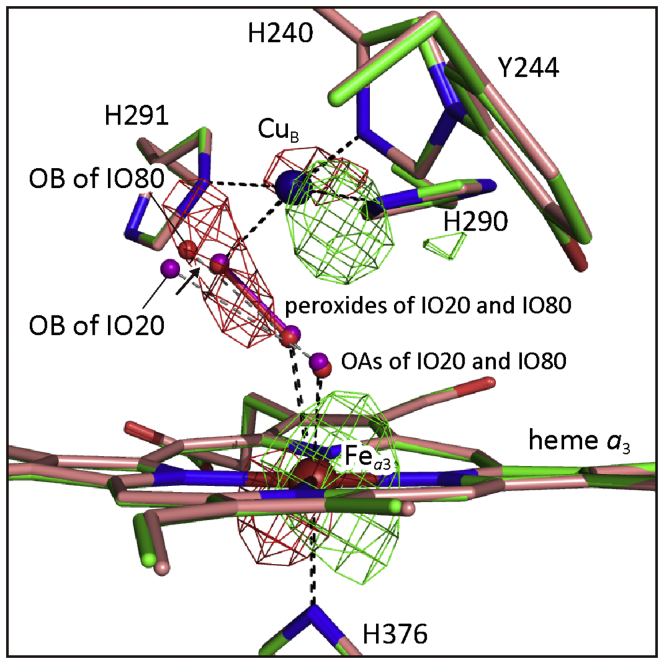
Figure 9.
Fo(IO20)-Fo(IO80) electron density map calculated with IO80 phases in the O2reduction site. Nitrogens and oxygens in heme a3 and amino acid residues are drawn by dark blue and red sticks. Carbons in IO80 and IO20 are beige and green sticks, respectively. Small purple and red spheres are nonbonding-oxygen atoms of IO80 and IO20, respectively. Two pairs of oxygen atoms linked with purple and red sticks denote the two peroxides shared by IO20 and IO80, respectively. Positive and negative densities are drawn at 4.0 σ by green and red cages, respectively. A pair of positive and negative peaks at each heavy atom site are consistent with a small (~0.06 Å) translational shift of CcO molecules upon transition from IO20 to IO80. A black arrow denotes the direction of migration of OB upon O→E transition.