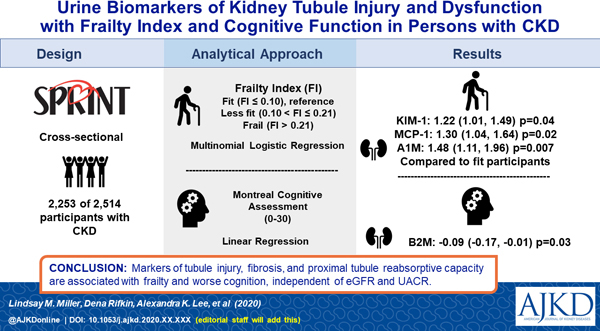
Abstract
Rationale and Objective:
The associations of glomerular markers of kidney disease (eGFR and albuminuria) with frailty and cognition are well established. However, the relationship of kidney tubular injury and dysfunction with frailty and cognition are unknown.
Study Design:
Observational cross-sectional study.
Setting & Participants:
2,253 participants with eGFR < 60 ml/min/1.73m2 in the Systolic Blood Pressure Intervention Trial
Exposures:
Eight urine biomarkers: Interleukin-18 [IL-18, pg/mL], kidney injury molecule-1 [KIM-1, pg/mL], neutrophil gelatinase-associated lipocalin [NGAL, ng/mL], chitinase-3-like protein-1 [YKL-40, pg/mL], monocyte chemoattractant protein-1 [MCP-1, pg/mL], α−1 microglobulin [α1M mg/g], beta-2 microglobulin [β2M ng/mL], and uromodulin [Umod ng/mL].
Outcomes:
Frailty was measured using a previously validated frailty index (FI), categorized as fit (FI ≤ 0.10), less fit (0.10 < FI ≤ 0.21) and frail (FI > 0.21). Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA).
Analytical Approach:
Associations between kidney tubule biomarkers with categorical FI were evaluated using multinomial logistic regression with the fit group as the reference. Cognitive function was evaluated using linear regression. Models were adjusted for demographic, behavioral and clinical variables including eGFR and urine albumin.
Results:
Three of the 8 urine biomarkers of tubule injury and dysfunction were independently associated with FI. Each two-fold higher level of urine KIM-1, a marker of tubule injury, was associated with a 1.22 [95% CI: 1.01, 1.49) fold greater odds of being in frail group. MCP-1, a marker of tubulo-interstitial fibrosis, was associated with a 1.30 [95% CI 1.04, 1.64] greater odds of being in frail group, and α1M, a marker of tubule re-absorptive capacity, was associated with a 1.48 [95% CI 1.11, 1.96] greater odds. These associations were independent of confounders including eGFR and urine albumin, and were stronger than those of urine albumin with frailty index (1.15 [95% CI 0.99, 1.34]). Higher urine β2M, another marker of tubule reabsorptive capacity, was associated with worse cognitive scores at baseline (β: ‒0.09; 95% CI ‒0.17, ‒0.01). Urine albumin was not associated with cognitive function.
Limitations:
Cross-sectional design, FI may not be generalizable in other populations.
Conclusions:
Urine biomarkers of tubule injury, fibrosis and proximal tubule reabsorptive capacity are variably associated with FI and worse cognition, independent of glomerular markers of kidney health. Future studies are needed to validate these results among other patient populations.
Index words: urine biomarkers, frailty, cognition, tubule injury and dysfunction
Graphical Abstract
PLAIN-LANGUAGE SUMMARY
The relationship of kidney tubule health with cognition and frailty
Standard measures of kidney function, including eGFR and albuminuria do not reliably capture the health of the kidney tubules, which may provide additional insight into the relationship between kidney function and cognition and frailty among older adults. We evaluated the relationship between 8 urine markers of kidney tubule function and injury with cognitive function and frailty among 2253 participants with chronic kidney disease in the Systolic Blood Pressure Intervention Trial. We found that participants with higher concentrations of urine KIM-1, MCP-1, and α1M were more likely to be categorized as frail. We also found that participants with higher concentrations of urine β2M had lower cognitive scores. These associations were significant beyond adjustment for eGFR and urine albumin.
Introduction
Chronic kidney disease (CKD) is highly prevalent in the US and world-wide, and is strongly associated with risk of kidney failure, cardiovascular disease, and all-cause mortality. Prior studies also consistently demonstrated that CKD is associated with frailty and worse cognitive function.1–6 However, these studies largely have utilized estimated glomerular filtration rate (eGFR) and urine albumin to creatinine ratio (albuminuria [ACR]), markers of glomerular function and injury, respectively, to characterize kidney health. These biomarkers do not account for the health of kidney tubules, a substantial limitation as, despite kidney tubule atrophy and fibrosis on biopsy being strongly associated with risk of kidney failure, these findings are poorly correlated to eGFR and ACR.7–11 Kidney tubules are critical for key physiological processes of the kidney, including endocrine resistance, reabsorption of key nutrients, elimination of toxins and drugs, and acid/base homeostasis.
A number of urine biomarkers have emerged that allow noninvasive assessment of kidney tubule injury and dysfunction; these include markers of tubule injury (Interleukin-18 [IL-18], kidney injury molecule-1 [KIM-1], and neutrophil gelatinase-associated lipocalin [NGAL]), repair (chitinase-3-like protein-1 [YKL-40]), and fibrosis (monocyte chemoattractant protein-1 [MCP-1]). Additional biomarkers include α−1 microglobulin [α1M] and beta-2 microglobulin [β2M], which reflect proximal tubule reabsorptive capacity, and uromodulin [Umod], which measures defense from infections and kidney tubule protein synthetic capacity. Prior studies from the Systolic Blood Pressure Intervention Trial (SPRINT) have documented strong associations between these biomarkers with risks of cardiovascular disease (CVD), acute kidney injury, and CKD progression.12–15 A recent study in SPRINT found that declining kidney function measured by eGFR was associated with an increased risk of MCI and probable dementia.16 While the biological mechanisms that underlie the shared pathophysiology between kidney tubule dysfunction and injury with frailty and cognitive function are unclear, it is possible that vulnerability to microvascular injury caused by hypertension, hypoxia from dysregulated cerebral blood flow, as well as chronic inflammation and endothelial dysfunction may play a role.17–20 Thus, we hypothesized that abnormalities in kidney tubule health may be associated with frailty index and cognition, above and beyond eGFR and ACR.
Methods
Study Sample
SPRINT is a randomized clinical trial that evaluated intensive (<120 mmHg) versus standard (<140 mmHg) blood pressure treatment targets in 9,361 hypertensive adults enrolled between November 2010 and March 2013 and followed until August 2015 with a median follow-up of 3.26 years. Details of the trial design have been described elsewhere.21 Briefly, participants were required to be at least 50 years of age, to have a SBP of 130 to 180 mmHg, and to be at elevated risk of cardiovascular events including at least one of the following: prevalent subclinical or clinical cardiovascular disease, chronic kidney disease with an eGFR between 20 and ≤ 60 ml per minute per 1.73 m2 estimated with the 4-variable Modification of Diet in Renal Disease (MDRD) equation, ≥ 75 years of age, or 10-year Framingham risk score of ≥ 15%. Important exclusion criteria include prior stroke, diabetes, and proteinuria > 1 g/day. The primary cardiovascular and cognitive results of SPRINT have been published elsewhere.21,22 The SPRINT study protocol was approved by Institutional Review Boards at each trial site, and all participants provided informed consent.
This ancillary study focuses on the contribution of kidney tubule dysfunction on clinical outcomes in the 2,514 SPRINT participants with CKD defined by estimated glomerular filtration rate (eGFR) <60 mL/minute per 1.73 m2 using the CKD-EPI combined creatinine and cystatin C equation.23 We measured the panel of kidney tubule dysfunction and injury markers in spot urine samples obtained at SPRINT baseline. After excluding 225 participants with missing urine specimens, 11 participants with missing frailty data, and 25 with missing cognitive data, the resulting analytic sample of 2,253 individuals were used for this analysis.
Biomarker measurement
All urine specimens were stored at −80°C until time of measurement in 2018. IL-18, KIM-1, MCP-1, and YKL-40 were measured together using a multiplex assay on a MESO Scale Diagnostics platform (Rockville, MD). Interassay CVs were 4.9% to 13.7%, 6.1% to 13.0%, 7.1% to 12.0%, and 6.5% to 11.1%, respectively. The analytic ranges were 2 to 10,000 pg/mL for IL-18, 4 to 200,000 pg/mL for KIM-1, 3 to 10,000 pg/mL for MCP-1, and 10 to 500,000 ng/mL for YKL-40. α-1 microglobulin (A1M) was measured using a Siemens nephelometric assay (Tarrytown, NY) with interassay CV of 3.5% to 8.8% and detectable range of 5 to 480 mg/g. β-2 microglobulin (B2M), Umod, and NGAL were measured on a multiplex assay on a MESO Scale Diagnostics platform (Rockville, MD) with interassay CVs of 15% to 16%, 13% to 16%, and 11% to 19%, respectively. The ranges of detection were 1.2 to 5020 ng/mL for B2M, 0.6 to 2510 ng/mL for uromodulin, and 6 to 251000 ng/mL for NGAL.13 Urine albumin (mg/L) and urine creatinine were measured using a nephelometric method (Siemens, Tarrytown, NY),24 and by an enzymatic procedure (Roche, Indianapolis, IN), respectively.
Outcomes
We categorized participants’ frailty status at baseline using a previously developed frailty index (FI) in SPRINT, based upon the model of deficit accumulation and derived from the African American Health (AAH) Study and the Hypertension in the Very Elderly Trial (HYVET) frailty indices.25,26 Briefly, the FI is comprised of a total of 36 items, including information on global cognitive function, self-rated health, self-rated depression, laboratory measurements, blood pressure (BP), and comorbid conditions. The FI is calculated as the sum of the score for each deficit divided by the total number of non-missing items, with scores ranging from 0.007 – 0.559 in SPRINT. For these analyses, we modified the FI omitting the item regarding CKD (based on eGFR), reducing the FI to 35 items. We then categorized frailty status as in previous work from SPRINT,25 with participants classified as fit (FI ≤ 0.10), less fit (0.10 < FI ≤ 0.21) or frail (FI > 0.21). The FI in SPRINT was shown to be associated with falls and all-cause hospitalizations,25 as well as greater risks of CVD and all-cause mortality.27
Cognitive function was measured at baseline using 3 validated screening tests. The Montreal Cognitive Assessment (MoCA; range 0–30) measures global cognitive function, the digit symbol coding (DSC, range 0–135) test measures attention and processing speed, and logical memory (LM) I and 2 (LM1, range 0–28; LM2, range 0–14) measures episodic verbal memory. Specifically, LM1 measures immediate recall, whereas LM2 measures delayed recall. Both the DSC and LM are subsets of the Wechsler Adult Intelligence Scale-IV. We also considered a MoCA score of < 24 to indicate screening positive for potential cognitive impairment.28
Covariates:
Potential confounders were measured at baseline and chosen a priori based on known risk factors for kidney disease, frailty, and cognitive function. They included age, sex, race (white/other vs Black), insurance status (Medicare [reference], uninsured, Medicaid, VA, private/other), body mass index (weight (kg)/height(m)2), years of education (less than a high school diploma, high school diploma, some college or greater), any alcohol use in the last 12 months (yes, no), current smoking (yes, no), baseline systolic BP (SBP; mmHg) and diastolic BP (DBP; mmHg), number of hypertension medications, urine creatinine (mg/g), cystatin C-creatinine based eGFR (mL/min/1.73 m2) using CKD-EPI equation and depressive symptoms using the 9-item patient health questionnaire (PHQ-9, range 0–27).23,29
Statistical Analysis
Biomarkers were log2 transformed to allow interpretation as “per two-fold higher” and to allow comparison of strengths of associations across biomarkers. Samples with biomarker values below the limit of detection were assigned a value equivalent to the lower limit of detection divided by the square root of two. All models were adjusted for urine creatinine to correct for urine tonicity. This was done instead of indexing due to the susceptibility of bias by muscle mass and health status.30 Descriptive statistics were performed on all participants and stratified by frailty category, as well as characteristics for participants with MoCA < 24, and included a correlation matrix for the biomarkers.
We used linear regression to evaluate kidney biomarkers with FI as a continuous outcome, and multinomial logistic regression to evaluate kidney biomarkers and FI as a categorical outcome, setting the fit group as the reference category. As a sensitivity analysis, we also evaluated the association with the less fit group as the reference. Cross-sectional analyses were evaluated with three models for each biomarker. We first evaluated the unadjusted association including only the biomarker of interest and urine creatinine. A second model adjusted for all confounders listed above except eGFR or urine albumin. Our final model additionally adjusted for eGFR and log2 urine albumin. Although our focus was on the biomarkers of kidney tubule injury and dysfunction, we evaluated the independent association with urine albumin (per 2-fold higher) and outcome measures in companion analyses, in order to provide an indicator for strengths of association compared to the other urine markers, as urine albumin is already available to clinicians in clinical practice. These models were repeated using the cognitive function tests using linear regression for MoCA continuously, and logistic regression using the cut point of <24 versus higher. We used the same modeling approach for cognitive function as we did for frailty. We used simple linear regression when evaluating continuous DSC test and LM tests.
All analyses were performed in Stata (version 15.1, Stata Corporation). Significance was defined for all analyses as p <0.05.
Results
Among 2,253 participants with eGFR < 60 ml/min/1.73m2 and complete biomarker, FI and cognitive data, 806 (36%) participants were considered frail, 1196 (53%) were less fit, and 251 (11%) were fit, and 1298 (58%) had a MoCA score of < 24. Age was similar across FI groups, whereas those classified as frail were more often women, black, had lower educational attainment, more often smoked, and had lower eGFR and urine ACR than those who were fit. Median urine concentrations of biomarkers of tubule function at baseline were consistently higher in those classified as frail than those classified as fit, with the exception of urine Umod which had the opposite pattern (Table 1). Characteristics between participants with missing data versus the total population can be found in table S1. Briefly, there was a greater percentage of black participants and participants treated by diuretics with missing data compared to the total population, and median concentrations of MCP-1, and β2M were higher (Table S1). A correlation matrix indicated that the urine tubule biomarkers were moderately correlated with one another with correlations ranging from ‒0.0007 to 0.81 (Table S2).
Table 1.
Characteristics of 2253 SPRINT participants with CKD stratified by Frailty Index and Cognitive Status.
| Characteristics | Fit (FI < 0.10) (n=251) |
Less Fit (0.10 > FI ≤
0.21) (n=1196) |
Frail (FI > 0.21) (n=806) |
MoCA < 24 (n=1298) |
|---|---|---|---|---|
|
Mean ±
SD
|
||||
| Age, years | 73.8 (8.8) | 73.4 (8.6) | 72.7 (9.7) | 74.7 (8.8) |
| Male Sex | 163 (65) | 737 (62) | 449 (56) | 813 (63) |
| Black Race | 54 (22) | 263 (22) | 247 (31) | 396 (31) |
| Some College or Greater | 196 (78) | 896 (75) | 505 (63) | 505 (63) |
| Body mass index, kg/m2 | 27.6 (4.6) | 29.3 (5.5) | 30.5 (6.5) | 29.4 (5.8) |
| Current Smoking | 5 (5) | 82 (14) | 111 (21) | 113 (16) |
| Depression Score | 0.75 (1.5) | 1.9 (2.5) | 5.5 (5.1) | 3.0 (3.9) |
| eGFR, mL/min/1.73m2 | 49.5 (8.5) | 46.9 (9.9) | 43.4 (11.1) | 45.7 (10.5) |
| Urine ACR, mg/g | 64.4(200.3) | 64.3 (154.3) | 105.2 (294.0) | 84.6 (237.0) |
| Urine creatinine, mg/g | 124.6 (76.3) | 124.4 (72.8) | 123.9 (74.8) | 124.4 (70.9) |
| SBP, mmHg | 138.5 (14.0) | 139.1 (16.1) | 140.9 (17.2) | 140.2 (16.2) |
| DBP, mmHg | 74.2 (9.6) | 74.0 (11.9) | 75.1 (13.3) | 73.5 (12.3) |
| Treated by diuretic | 112 (45) | 644 (54) | 455 (56) | 694 (54) |
| Treated by ARB or ACEi | 153 (61) | 750 (63) | 501 (62) | 792 (61) |
| LDL Cholesterol | 106.3 (26.6) | 107.5 (33.4) | 103.5 (38.2) | 105.4 (33.9) |
| Biomarkers | Median (IQR) | |||
| IL-18, pg/mL | 27.6 (15.7, 48.1) | 30.1 (15.9, 55.6) | 33.3 (17.4, 60.2) | 31.0 (16.8, 56.8) |
| NGAL, ng/mL | 24.2 (13.6, 50.2) | 26.9 (14.6, 53.6) | 31.0 (16.2, 66.5) | 29.3 (15,7, 61.1) |
| YKL-40, pg/mL | 590.8 (248.4, 1235.6) | 528.5 (217.7, 1120.9) | 585.9 (214.6, 1476.8) | 568.6 (221.7, 1334.0) |
| KIM-1, pg/mL | 741.9 (294.3, 1279.9) | 850.3(384.9, 1574.8) | 918.6 (425.0, 1737.2) | 846.3 (416.5, 1586.3) |
| MCP-1, pg/mL | 153.7 (80.1, 287.3) | 179.0 (89.0, 318.9) | 188.5 (98.0, 346.5) | 183.9 (96.2, 326) |
| α1M, mg/g | 12.8 (6.6, 21.9) | 12.9 (7.2, 24.4) | 14.7 (7.9, 28.0) | 14.5 (7.6, 25.8) |
| β2M, ng/mL | 104.2(44.0, 327.3) | 96.0 (38.1, 293.2) | 109.1 (37.6, 382.0) | 114.2 (43.0, 342.0) |
| Umod, ng/mL | 6.9 (4.8, 9.9) | 7.0 (4.6, 10.4) | 5.9 (3.9, 9.5) | 6.6 (4.5, 9.9) |
| Urine Albumin, mg/L | 12.0 (6.0, 42.0) | 15.0 (7.0, 41.0) | 21.0 (8.0, 73.0) | 17.0 (8.0, 50.0) |
Note. Values for continuous variables are given as mean ± standard deviation; those for categorical variables, as count (percentage).
Abbreviations: eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; ARB, angiotensin II receptor blocker; ACEi, angiotensin-converting enzyme inhibitor; LDL, low-density lipoprotein; IL-18, interleukin-18; KIM-1, kidney injury molecule 1; NGAL, neutrophil gelatinase-associated lipocalin; MCP-1, monocyte chemoattractant protein 1; β2M, β−2 microglobulin; α1M, α−1 microglobulin; Umod, uromodulin.
Associations of Kidney Tubule Dysfunction and Injury with Frailty Index
In unadjusted analyses, 6 of the 8 biomarkers of kidney tubule injury and dysfunction were statistically significantly associated with the continuous FI, implicating worse kidney tubule health with worse FI scores (higher urine concentrations of all biomarkers except Umod). The exceptions were YKL-40 and β2M which were not associated with FI. In adjusted models, higher urine KIM-1, MCP-1, α1M, and albumin remained significantly associated with continuous FI scores. Similarly, when evaluating the categorical FI outcome (fit, less fit, and frail) in unadjusted models, the same 6 biomarkers were statistically significantly associated with frailty, relative to fit participants. In adjusted models without eGFR or albuminuria, higher urine NGAL, KIM-1, MCP-1, α1M, and β2M, and lower Umod were all statistically associated with higher likelihood of being in the frail group. The addition of eGFR and urine albumin attenuated associations, such that KIM-1, MCP-1, α1M remained statistically significantly associated with being frail compared with fit in fully adjusted models. As a comparator, higher urine albumin was also associated with FI in this model; however, the associations of KIM-1, MCP-1 and α1M appeared stronger than urine albumin, even though these biomarkers had already been adjusted for urine albumin concentrations (Table 2, Figure 1). In our sensitivity analysis evaluating the association using the less fit group as the reference, effect sizes were attenuated compared to odds ratios with the fit group as the reference, and only MCP-1 and α1M remained statistically significantly associated (Table S3).
Table 2.
Cross-sectional Association between Urine Biomarkers of Kidney Tubule Injury and Dysfunction and Frailty in 2253 SPRINT Participants with CKD
| Frailty Index | ||||
|---|---|---|---|---|
| Biomarker | Frailty Continuous (range 0.007 – 0.559) | Fit (FI ≤ 0.10) | Less fit (0.10 < FI ≤ 0.21) | Frail (FI > 0.21) |
| (range 0.007 – 0.559) | OR (95% CI) | |||
| Log 2 IL-18, pg/mL | ||||
| Unadjusted | 0.01 (0.002, 0.01)*** | - | 1.05 (0.94, 1.18) | 1.17 (1.04, 1.32)* |
| Adjusteda | 0.003 (−0.0004, 0.01) | - | 1.06 (0.85, 1.31) | 1.12 (0.89, 1.41) |
| Adjusted + eGFR & albuminb | 0.002 (−0.002, 0.005) | - | 1.00 (0.79, 1.28) | 1.06 (0.82, 1.37) |
| Log 2 NGAL, ng/mL | ||||
| Unadjusted | 0.003 (0.001, 0.01)** | - | 1.05 (0.96, 1.14) | 1.13 (1.03, 1.24)** |
| Adjusteda | 0.001 (−0.001, 0.003) | 1.21 (1.01, 1.44)* | 1.23 (1.02, 1.49)* | |
| Adjusted + eGFR & albumin | −0.001 (−0.003, 0.002) | 1.14 (0.95, 1.37) | 1.13 (0.93, 1.38) | |
| Log 2 YKL-40, pg/mL | ||||
| Unadjusted | 0.001 (−0.0001, 0.003) | - | 0.97 (0.91, 1.04) | 1.01 (0.84, 1.08) |
| Adjusteda | 0.0003 (−0.002, 0.002) | 0.99 (0.87, 1.12) | 0.99 (0.86, 1.13) | |
| Adjusted + eGFR & albumin | 0.0002 (−0.002, 0.002) | 0.98 (0.85, 1.13) | 0.99 (0.85, 1.15) | |
| Log 2 KIM-1, pg/mL | ||||
| Unadjusted | 0.01 (0.003, 0.01)*** | - | 1.10 (1.01, 1.20)* | 1.19 (1.09, 1.31)*** |
| Adjusteda | 0.01 (0.003, 0.01)*** | 1.16 (0.99, 1.34) | 1.36 (1.14, 1.63)** | |
| Adjusted + eGFR & albuminb | 0.004, (0.001, 0.01)** | 1.09 (0.91, 1.30) | 1.22 (1.01, 1.49)* | |
| Log 2 MCP-1, pg/mL | ||||
| Unadjusted | 0.01 (0.003, 0.01)*** | - | 1.11 (0.99, 1.23) | 1.24 (1.10, 1.39)*** |
| Adjusteda | 0.01 (0.002, 0.01)*** | 1.12 (0.93, 1.36) | 1.37 (1.10, 1.69)** | |
| Adjusted + eGFR & albuminb | 0.004 (0.001, 0.01)** | 1.08 (0.87, 1.33) | 1.30 (1.04, 1.64)* | |
| Log 2 α1M, mg/g | ||||
| Unadjusted | 0.01 (0.005, 0.01)*** | - | 1.06 (0.94, 1.19) | 1.25 (1.11, 1.42)*** |
| Adjusteda | 0.01 (0.01, 1.02)*** | 1.41 (1.13, 1.75)** | 1.91 (1.50, 2.43)*** | |
| Adjusted + eGFR & albuminb | 0.01 (0.004, 0.01)*** | 1.18 (0.91, 1.54) | 1.48 (1.11, 1.96)** | |
| Log 2 β2M, ng/mL | ||||
| Unadjusted | 0.0004 (−0.0001, 0.002) | - | 0.98 (0.93, 1.04) | 1.00 (0.95, 1.06) |
| Adjusteda | 0.002 (0.0003, 0.003)* | 1.07 (0.98, 1.18) | 1.11 (1.00, 1.22)* | |
| Adjusted + eGFR & albuminb | 0.0004 (−0.001, 0.002) | 1.03 (0.93, 1.14) | 1.02 (0.92, 1.14) | |
| Log 2 Umod, ng/mL | ||||
| Unadjusted | −0.01 (−0.01, −0.01)*** | - | 0.99 (0.85, 1.15) | 0.80 (0.68, 0.93)** |
| Adjusteda | −0.01 (−0.01, −0.003)** | 1.04 (0.78, 1.38) | 0.90 (0.66, 1.22) | |
| Adjusted + eGFR & albuminb | −0.002 (−0.01, 0.002) | 1.26 (0.92, 1.73) | 1.30 (0.93, 1.82) | |
| Log 2 albumin, mg/L | ||||
| Unadjusted | 0.005 (0.003, 0.01)*** | - | 1.05 (0.98, 1.13) | 1.16 (1.08, 1.25)*** |
| Adjusteda | 0.005 (0.003, 0.01)*** | 1.20 (1.05, 1.37)** | 1.31 (1.14, 1.51)*** | |
| Adjusted + eGFR | 0.003 (0.001, 0.004)** | 1.12 (0.97, 1.29) | 1.15 (0.99, 1.34) | |
|
| ||||
Note.IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; MCP-1, monocyte chemoattractant protein-1; β2M, β2-microglobulin; α1M, α1-microglobulin; Umod, uromodulin.
Adjusted for age, race, sex, body mass index, alcohol use, years of education, insurance status, baseline systolic and diastolic blood pressure, smoking status, and urine creatinine.
Adjusted for all variables listed above and estimated glomerular filtration rate (eGFR) and Log2 albumin.
P<0.05;
P<0.01;
P<0.001
Figure 1.
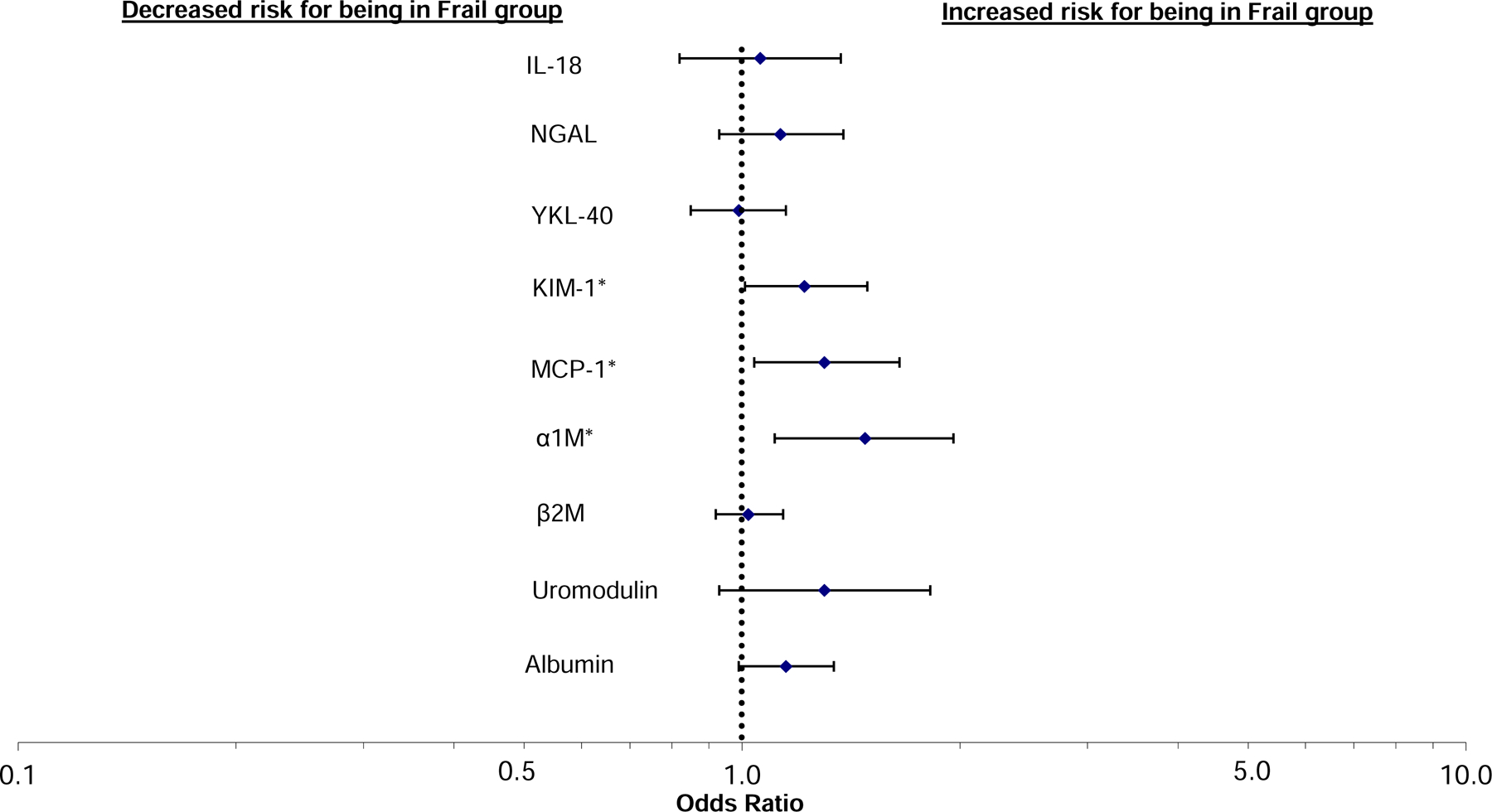
Multinomial regression showing the baseline association of urine biomarkers of kidney tubule injury and dysfunction with frailty index status (each modeled as “per 2-fold higher” with frailty compared with fit older adults [less fit group omitted]). Models were adjusted for age, sex, race, BMI, alcohol use, years of education, insurance status, SBP and DBP, smoking status, urine creatinine, eGFR, and albuminuria. IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; MCP, monocyte chemoattractant protein-1; β2M, β2-microglobulin; A1M, α1-microglobulin; Umod, uromodulin.
Association of Kidney Tubule Dysfunction and Injury with Cognitive Function
When evaluating the unadjusted association between biomarkers of kidney tubule health with cognitive function measured by MoCA as a continuous variable, we found that each 2-fold higher urine NGAL, YKL-40, KIM-1, MCP-1 and β2M were statistically significantly associated with lower cognitive scores. However, only higher β2M remained statistically significantly associated with worse cognition after adjustment for confounders, eGFR and urine albumin (Table 3). Urine albumin was not associated with cognitive scores. When evaluated as a binary variable (MoCA < 24), none of the biomarkers were associated lower cognitive function (Table 3). In companion analyses we evaluated the DSC and LM2 as more specific measures of cognitive function. Higher urine NGAL and β2M were statistically associated with lower DSC scores in fully adjusted models, and Umod was statistically associated with worse LM2 scores (Table 4).
Table 3.
Cross-Sectional Association between Urine Biomarkers of Kidney Tubule Injury and Dysfunction and Cognitive Function in 2253 SPRINT Participants with CKD
| Biomarker | MoCA Score |
MoCA Score < 24 |
|---|---|---|
| (Range 5–30) | (n=1298, 58%) | |
| β coefficient (95% CI) | OR (95% CI) | |
| Log 2 IL-18, pg/mL | ||
| Unadjusted | −0.11 (−0.26, 0.04) | 1.05 (0.98, 1.13) |
| Adjusteda | −0.04 (−0.22, 0.14) | 1.04 (0.93, 1.17) |
| Adjusted + eGFR & albuminb | −0.04 (−0.22, 0.15) | 1.05 (0.93, 1.19) |
| Log 2 NGAL, ng/mL | ||
| Unadjusted | −0.11 (−0.22, −0.01)* | 1.05 (0.99, 1.10) |
| Adjusteda | −0.04 (−0.18, 0.10) | 1.02 (0.93, 1.11) |
| Adjusted + eGFR & albumin | −0.03 (−0.17, 0.11) | 1.02 (0.93, 1.12) |
| Log 2 YKL-40, pg/mL | ||
| Unadjusted | −0.11 (−0.19, −0.02)* | 1.04 (1.00, 1.08) |
| Adjusteda | −0.01 (−0.12, 0.09) | 0.99 (0.93, 1.06) |
| Adjusted + eGFR & albumin | −0.01 (−0.12, 0.10) | 0.99 (0.92, 1.06) |
| Log 2 KIM-1, pg/mL | ||
| Unadjusted | −0.11 (−0.23, 0.01) | 1.07 (1.01, 1.14)* |
| Adjusteda | −0.13 (−0.27, 0.02) | 1.06 (0.96, 1.16) |
| Adjusted + eGFR & albuminb | −0.12 (−0.28, 0.03) | 1.07 (0.97, 1.18) |
| Log 2 MCP-1, pg/mL | ||
| Unadjusted | −0.29 (−0.43, −0.14)*** | 1.15 (1.07, 1.23)*** |
| Adjusteda | −0.14 (−0.30, 0.03) | 1.08 (0.97, 1.20) |
| Adjusted + eGFR & albuminb | −0.13 (−0.31, 0.04) | 1.08 (0.97, 1.21) |
| Log 2 α1M, mg/g | ||
| Unadjusted | −0.26 (−0.41, −0.11)** | 1.12 (1.04, 1.21)** |
| Adjusteda | −0.10 (−0.28, 0.07) | 1.02 (0.91, 1.14) |
| Adjusted + eGFR & albuminb | −0.08 (−0.29, 0.12) | 1.03 (0.91, 1.18) |
| Log 2 β2M, ng/mL | ||
| Unadjusted | −0.11 (−0.17, −0.04)** | 1.04 (1.01, 1.07)* |
| Adjusteda | −0.09 (−0.17, −0.01)* | 1.04 (0.99, 1.10) |
| Adjusted + eGFR & albuminb | −0.09 (−0.17, −0.01)* | 1.05 (0.99, 1.10) |
| Log 2 Umod, ng/mL | ||
| Unadjusted | 0.08 (−0.11, 0.27) | 1.04 (0.95, 1.14) |
| Adjusteda | −0.13 (−0.35, 0.10) | 1.11 (0.96, 1.29) |
| Adjusted + eGFR & albuminb | −0.19 (−0.43, 0.05) | 1.12 (0.96, 1.31) |
| Log 2 urine albumin, mg/L | ||
| Unadjusted | −0.15 (−0.23, −0.06)** | 1.05 (1.01, 1.09)* |
| Adjusteda | −0.03 (−0.13, 0.07) | 1.00 (0.93, 1.06) |
| Adjusted + eGFR | −0.02 (−0.12, 0.09) | 1.00 (0.93, 1.07) |
Note.IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; MCP, monocyte chemoattractant protein-1; β2M, β2-microglobulin; A1M, α1-microglobulin; Umod, uromodulin. Unadjusted models include urine creatinine
Adjusted for age, race, sex, body mass index, alcohol use, years of education, insurance status, baseline systolic and diastolic blood pressure, smoking status, and urine creatinine.
Adjusted for all variables listed above and estimated glomerular filtration rate (eGFR) and Log2 albumin.
Table 4:
Cross-sectional Association between Urine Biomarkers of Kidney Tubule Injury and Dysfunction and Measures of Cognitive Function in 2253 SPRINT Participants with CKD
| Biomarker | Digit Symbol Coding (range 0–135) | Logical Memory Test 1 (Immediate Recall) (range 0–28) | 2 (Delayed Recall) (range 0–14) |
|---|---|---|---|
|
B coefficient (95%
CI)
|
|||
| Log 2 IL-18, pg/mL | |||
| Unadjusted | −0.58 (−1.09, −0.07)* | −0.12 (−0.29, 0.06) | −0.04 (−0.16, 0.08) |
| Adjust0EDA | −0.001 (−0.60, 0.59) | −0.07 (−0.31, 0.16) | −0.01 (−0.17, 0.15) |
| Adjusted + eGFR & albuminb | 0.04 (−0.59, 0.67) | −0.10 (−0.35, 0.15) | −0.01 (−0.18, 0.16) |
| Log 2 NGAL, ng/mL | |||
| Unadjusted | −0.74 (−1.12, −0.37)*** | −0.10 (−0.23, 0.03) | −0.03 (−0.12, 0.06) |
| Adjusteda | −0.53 (−0.98, −0.07) | −0.11 (−0.28, 0.07) | −0.04 (−0.16, 0.08) |
| Adjusted + eGFR & albumin | −0.49 (−0.96, −0.02)* | −0.11 (−0.29, 0.07) | −0.04 (−0.16, 0.09) |
| Log 2 YKL-40, pg/mL | |||
| Unadjusted | −0.60 (−0.89, −0.30)*** | −0.07 (−0.18, 0.03) | −0.02 (−0.09, 0.05) |
| Adjusteda | −0.24 (−0.60, 0.11) | −0.01 (−0.15, 0.13) | −0.03 (−0.12, 0.07) |
| Adjusted + eGFR & albumin | −0.27 (−0.63, 0.10) | −0.02 (−0.17, 0.12) | −0.03 (−0.13, 0.07) |
| Log 2 KIM-1, pg/mL | |||
| Unadjusted | −0.27 (−0.69, 0.15) | −0.06 (−0.21, 0.08) | −0.11 (−0.21, 0.01) |
| Adjusteda | −0.47 (−0.96, 0.02) | 0.02 (−0.17, 0.21) | −0.11 (−0.23, 0.03) |
| Adjusted + eGFR & albuminb | −0.41 (−0.92, 0.11) | 0.03 (−0.17, 0.23) | −0.10 (−0.24, 0.03) |
| Log 2 MCP-1, pg/mL | |||
| Unadjusted | −1.12 (−1.61, −0.63)*** | −0.32 (−0.49, −0.15)*** | −0.27 (−0.38, −0.15)*** |
| Adjusteda | −0.56 (−1.13, 0.002) | −0.15 (−0.37, 0.08) | −0.15 (−0.30, 0.003) |
| Adjusted + eGFR & albuminb | −0.55 (−1.14, 0.03) | −0.16 (−0.39, 0.07) | −0.15 (−0.31, 0.004) |
| Log 2 α1M, mg/g | |||
| Unadjusted | −0.91 (−1.43, −0.39)** | −0.37 (−0.55, −0.19)*** | −0.31 (−0.43, −0.19)*** |
| Adjusteda | −0.19 (−0.78, 0.41) | −0.12 (−0.35, 0.11) | −0.07 (−0.23, 0.08) |
| Adjusted + eGFR & albuminb | 0.09 (−0.60, 0.77) | −0.12 (−0.39, 0.15) | −0.07 (−0.25, 0.12) |
| Log 2 β2M, ng/mL | |||
| Unadjusted | −0.43 (−0.67, −0.20)*** | −0.11 (−0.20, −0.03)** | −0.10 (−0.16, −0.04)*** |
| Adjusteda | −0.33 (−0.59, −0.07)* | −0.02 (−0.12, 0.08) | −0.04 (−0.11, 0.03) |
| Adjusted + eGFR & albuminb | −0.29 (−0.56, −0.02)* | −0.01 (−0.12, 0.10) | −0.03 (−0.11, 0.04) |
| Log 2 Umod, ng/mL | |||
| Unadjusted | 1.05 (−0.39, 1.71)** | −0.10 (−0.33, 0.13) | −0.14 (−0.30, 0.02) |
| Adjusteda | 0.04 (−0.73, 0.80) | −0.21 (−0.51, 0.09) | −0.30 (−0.50, −0.10)** |
| Adjusted + eGFR & albuminb | −0.25 (−1.07, 0.56) | −0.30 (−0.62, 0.01) | −0.37 (−0.58, −0.15)** |
| Log 2 albumin, mg/L | |||
| Unadjusted | −0.66 (−0.95, −0.36)*** | −0.17 (−0.28, −0.07)** | −0.14 (−0.21, −0.07)*** |
| Adjusteda | −0.18 (−0.52, 0.15) | −0.01 (−0.14, 0.12) | −0.02 (−0.11, 0.07) |
| Adjusted + eGFR & albuminb | −0.07 (−0.43, 0.28) | 0.02 (−0.12, 0.16) | −0.01 (−0.10, 0.09) |
Note. MoCA, Montreal Cognitive Assessment; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; MCP, monocyte chemoattractant protein-1; β2M, β2-microglobulin; A1M, α1-microglobulin; UMOD, uromodulin.
Adjusted for age, race, sex, body mass index, alcohol use, years of education, insurance status, baseline systolic and diastolic blood pressure, smoking status, and urine creatinine
Adjusted for all variables listed above and estimated glomerular filtration rate (eGFR) and Log2 albumin.
P<0.05;
P<0.01;
P<0.001
Regression coefficients for the full model evaluating β2M with MoCA scores, and odds ratios for the full model evaluating α1M with FI can be found in tables S4 and S5, respectively.
Discussion
In this study of 2,253 SPRINT participants with CKD, we found that higher concentrations of several urine markers of kidney tubule dysfunction and injury were modestly associated with frailty and cognitive dysfunction. The observed associations were independent of eGFR and urine albumin, and other CVD risk factors. When compared to urine albumin, urine α1M, MCP-1, KIM-1 had stronger associations with FI and worse cognitive function. While higher β2M was associated with lower MoCA scores, higher NGAL and β2M were associated with lower scores on the DSC, a test of attention and processing speed, and lower Umod was associated with lower scores on logical memory assessment. Overall, these findings suggest that markers of kidney tubule dysfunction and injury have the potential to identify individuals with higher burdens of frailty and cognitive dysfunction above and beyond glomerular markers of kidney health (eGFR and albuminuria).
Persons with CKD are at a substantially greater risk for a myriad of negative health outcomes including heart disease, stroke, acute kidney injury, and mortality, and previous studies have suggested the possibility that CKD also identifies individuals at higher risk for cognitive dysfunction and frailty.1–6,31–33 Until now, studies evaluating relationships of kidney disease with cognition and frailty have exclusively used measures of kidney health that capture glomerular function and injury. Both cross-sectional and longitudinal studies have reported statistically significant associations between worse glomerular function and injury and frailty. For instance, in a cross-sectional study in the Cardiovascular Health Study, Shlipak et al. found that the prevalence of frailty and disability was greater among participants with elevated serum creatinine level compared to participants with normal kidney function, and that frailty remained significantly associated with elevated serum creatinine after adjustment for comorbid conditions.34 Other studies reported associations of higher albuminuria with frailty.35 Results are similar in longitudinal studies. For example, Darsie et al. found that older adults with a eGFR < 60 had more rapid decline in cognitive function scores over 9 years of follow-up,36 while Kurella et al. found that that participants with an eGFR of < 45 had a significantly greater change in modified mini mental state exam scores (3MS) over follow-up.37 However, other studies have found no associations between CKD and cognitive function and frailty. A recent study by Scheppach et al. found that while albuminuria was associated with increased risk of incident dementia, only cystatin C based eGFR was statistically associated whereas eGFR by creatinine was not.38 Similarly, Zijlstra et al. found that only CKD stage 4 was statistically associated with cognitive decline, which was steeper among older adults with a history of vascular disease. They found no association with kidney disease and functional status.39 Still, these studies have focused on glomerular function and injury, and results have been mixed. While these are the standard clinical measurements of kidney function used in clinical practice, they only capture one aspect of kidney health. Therefore, we extend these findings by demonstrating that kidney tubule dysfunction and injury are associated with cognitive impairment, and that such relationships are evident for several of the biomarkers even after accounting for eGFR and albuminuria. Thus, the full extent of the relationship of kidney disease with cognitive impairment may have been underestimated in prior studies.
The mechanisms that underlie the associations between kidney tubular health with both frailty and cognitive impairment are uncertain and require additional study. While it is possible that there is a causal link between kidney disease and both frailty and cognition, we favor a hypothesis of systemic disease processes of vascular aging that may simultaneously affect the kidney, functional status, and cognition. We have previously shown that these biomarkers are associated with a greater risk of CVD,12 which has been consistently related to both frailty and worse cognition, suggesting these outcomes may share a common etiology. Both the kidney and the brain are highly vascular organs with tightly autoregulated blood flow in an effort to protect against vascular insults.17,18 Hypoxia, chronic inflammatory stress, and other mechanisms that promote vascular aging may lead to end-organ damage that is manifested clinically by impairments in kidney function, functional status, and cognition.19,20,40,41 Future studies to understand these potential mechanisms should be a high priority, as, ultimately, intervening in these pathways may improve multiple facets of aging concurrently.
In our study, we found that higher urine α1M was associated with FI, and higher β2M was associated with both worse global cognitive function and attention and processing speed. These proteins are freely filtered at the glomerulus, and then avidly reabsorbed in the proximal tubules, such that urinary excretion is low in healthy individuals. Drugs that damage proximal tubule reabsorptive capacity increase urine excretion of these proteins.42 Thus, higher concentrations in both markers suggest deficits in proximal tubular reabsorption capacity.43 This action of the proximal tubules is highly dependent on mitochondrial activity. Therefore, the consistency of these two markers with the endpoints investigated in this study suggest that mitochondrial dysfunction, hypoxia, or dysfunction in the proximal tubule of the kidney may be particularly important in defining the mechanisms linking kidney disease with frailty and cognitive impairment. On the other hand, we are uncertain why one marker would be associated with one outcome but not the other, and vice versa with the alternate marker. These findings require confirmation in other settings, and additional investigation.
Similarly, MCP-1 is expressed in epithelial and fibroblast cells in the kidney tubules. In persons with diabetes, higher urine MCP-1 has been linked with greater tubulo-interstitial fibrosis on kidney biopsy.44,45 We have shown that higher urine MCP-1 concentrations are associated with CVD events in kidney transplant recipients.46 Here, we found that higher MCP-1 was independently associated with FI. Thus, accelerated aging and systemic fibrotic processes may represent another potential pathway linking the observed associations. Finally, we also found that higher KIM-1 was associated with FI suggesting that injury to the proximal tubule may reflect tissue damage elsewhere in the body that might lead to frailty.
Strengths of this study include its relatively large sample size, evaluation of patients with established CKD, and availability of 8 markers of tubule dysfunction and injury concurrent with a validated frailty index and cognitive tests. The study also has important limitations. SPRINT excluded participants with diabetes mellitus, prior stroke, and proteinuria >1 g/day, all of which are linked with frailty and cognitive impairment. Additionally, clinical trial participants are likely to be less frail and cognitively impaired than the general population, which may have limited our ability to detect associations. Similarly, the FI may not generalize to other studies or patient populations, and future studies will be needed to validate these results. All participants with kidney tubule biomarker measurements had eGFR below 60 mL/min/1.73m2 at baseline in SPRINT, therefore it is unclear whether these associations will hold up in other populations. This study had a cross-sectional design, thus we are not able to assess if kidney tubule dysfunction preceded frailty or cognitive impairment or vice versa. Longitudinal studies to assess temporality will be an important next step. Furthermore, because we have suggested that the biological mechanism between tubular markers with cognitive function and frailty might lie on the same etiologic pathway as CVD, it is possible that clinical vascular disease is on the causal pathway to kidney tubule dysfunction and injury and each of these outcomes and may act as mediators. However, due to the cross-sectional nature of the analysis, and the lack of vascular/subclinical CVD variables available in SPRINT, we could not assess the degree to which subclinical vascular disease may help explain the observed association. Finally, there may be unmeasured/residual confounding in our models, however, we utilized available covariates known to be associated with the exposures and outcomes.
In conclusion, in adults with hypertension and CDK participating in SPRINT, several markers of kidney tubule dysfunction and injury, specifically higher KIM-1, MCP-1 and α1M, were modestly but statistically significantly associated with the FI. Similarly, higher NGAL, B2M and lower Umod were modestly but statistically associated with distinct measures of cognitive dysfunction. These associations were independent of eGFR, albuminuria, and other CKD risk factors, and consistently appeared stronger than associations of urine albumin with these same outcomes. While these results are hypothesis-generating, they suggest the possibility that kidney tubule injury, tubulo-interstitial fibrosis and deficits in proximal tubule reabsorptive capacity may share a common systemic pathology of vascular aging with mechanisms promoting frailty and cognitive impairment. Future studies are needed to validate these results among other patient populations.
Supplementary Material
Support:
Dr. Lindsay Miller was supported by a Ruth L. Kirschstein training grant from the National Institute of Diabetes and Digestive Kidney Diseases (NIDDK; T32DK104717). Dr. Joachim H. Ix was supported by mid-career mentoring award from the NIDDK (K24DK110427). The ancillary study measurements and data analysis were supported by an R01 award from the NIDDK to Drs. Ix and Shlipak (R01DK098234). Dr. Nicholas Pajewski was supported by R01AG055606, the Wake Forest Claude Pepper Center (P30AG021332), and the Alzheimer’s Association. Dr. Alexandra Lee was supported by T32AG000212. Dr. Kurella Tamura is supported by R01 DK092241 from NIDDK. The Systolic Blood Pressure Intervention Trial was funded by the National Institutes of Health (including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke) under contracts HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C and interagency agreement A-HL-13–002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. Azilsartan and chlorthalidone (combined with azilsartan) were provided by Takeda Pharmaceuticals International Inc. Additional support was provided through the following National Center for Advancing Translational Sciences clinical and translational science awards: UL1TR000439 (awarded to Case Western Reserve University); UL1RR025755 (Ohio State University); UL1RR024134 and UL1TR000003 (University of Pennsylvania); UL1RR025771 (Boston University); UL1TR000093 (Stanford University); UL1RR025752, UL1TR000073, and UL1TR001064 (Tufts University); UL1TR000050 (University of Illinois); UL1TR000005 (University of Pittsburgh); 9U54TR000017–06 (University of Texas Southwestern Medical Center); UL1TR000105–05 (University of Utah); UL1 TR000445 (Vanderbilt University); UL1TR000075 (George Washington University); UL1 TR000002 (University of California, Davis); UL1 TR000064 (University of Florida); and UL1TR000433 (University of Michigan); and by National Institute of General Medical Sciences, Centers of Biomedical Research Excellence award NIGMS P30GM103337 (awarded to Tulane University). The funders had no role in the study design, data collection, analysis, reporting or decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Deckers K, Camerino I, van Boxtel MP, et al. Dementia risk in renal dysfunction: a systematic review and meta-analysis of prospective studies. Neurology 2017;88(2):198–208. doi: 10.1212/WNL.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etgen T Kidney disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther 2015;7(1):29. doi: 10.1186/s13195-015-0115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Z, Ruan Q, Yu Z, Sun Z. Chronic kidney disease-related physical frailty and cognitive impairment: a systemic review. Geriatr Gerontol Int 2017;17(4):529–544. doi: 10.111/ggi.12758 [DOI] [PubMed] [Google Scholar]
- 4.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 2012;60(6):912–921. doi: 10.1053/j.ajkd.2012.05.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalrymple LS, Katz R, Rifkin DE, et al. Kidney function and prevalent and incident frailty. Clin J Am Soc Nephrol 2013;8(12):2091–2099. doi: 10.2215/CJN.02870313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado C, Grimes BA, Glidden DV, Shlipak M, Sarnak MJ, Johansen KL. Association of Frailty based on self-reported physical function with directly measured kidney function and mortality. BMC Nephrol 2015;16(1):203. doi: 10.1186/s12882-0.15-0.202-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong AC, Fine LG. Loss of glomerular function and tubulointerstitial fibrosis: Cause or effect? Kidney Int 1994;45(2):345–351. doi: 10.1038/ki.1994.44 [DOI] [PubMed] [Google Scholar]
- 8.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 1992;20(1):1–17. doi: 10.1016/S0272-6386(12)80312-X [DOI] [PubMed] [Google Scholar]
- 9.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 2010;152(9):561. doi: 10.7326/0003-4819-152-9-201005040-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howie AJ, Ferreira MAS, Adu D. Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant 2001;16(6):1163–1169. doi: 10.1093/ndt/16.61163 [DOI] [PubMed] [Google Scholar]
- 11.Takebayashi S, Kiyoshi Y, Hisano S, et al. Benign nephrosclerosis: incidence, morphology and prognosis. Clin Nephrol 2001;55(5):349–356. [PubMed] [Google Scholar]
- 12.Garimella PS, Lee AK, Ambrosius WT, et al. Markers of kidney tubule function and risk of cardiovascular disease events and mortality in the SPRINT trial. Eur Heart J 2019;40(42):3486–3493. doi: 10.1093/eurheartj/ehz392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AK, Katz R, Jotwani V, et al. Distinct Dimensions of Kidney Health and Risk of Cardiovascular Disease, Heart Failure, and Mortality. Hypertension 2019;74(4):872–879. doi: 10.1161/HYPERTENSIONAHA.119.13339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullen AL, Katz R, Lee AK, et al. The SPRINT trial suggests that markers of tubule cell function in the urine associate with risk of subsequent acute kidney injury while injury markers elevate after the injury. Kidney Int 2019;96(2):470–479. doi: 10.1016/j.kint.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malhotra R, Katz R, Jotwani V, et al. Urine Markers of Kidney Tubule Cell Injury and Kidney Function Decline in SPRINT Trial Participants with CKD. Clin J Am Soc Nephrol 2020;15(3):349–358. doi: 10.2215/CJN.02780319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurella Tamura M, Gaussoin SA, Pajewski NM, Chelune GJ, Freedman BI, Gure TR, Haley WE, Killeen AA, Oparil S, Rapp SR, Rifkin DE, Supiano M, Williamson JD, Weiner DE; SPRINT Research Group*. Kidney Disease, Intensive Hypertension Treatment, and Risk for Dementia and Mild Cognitive Impairment: The Systolic Blood Pressure Intervention Trial. J Am Soc Nephrol 2020. September;31(9):2122–2132. doi: 10.1681/ASN.2020010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mogi M, Horiuchi M. Clinical interaction between brain and kidney in small vessel disease. Cardiol Res Pract 2011;2011. doi: 10.4061/2011/306189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46(1):200–204. doi: 10.1161/01.HYP.0000168052.00426.65 [DOI] [PubMed] [Google Scholar]
- 19.Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, Langhoff E. Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab 2007;27(11):1861–1869. doi: 10.1038/sj.jcbfm.9600478 [DOI] [PubMed] [Google Scholar]
- 20.Khatri M, Wright CB, Nickolas TL, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). Stroke 2007;38(12):3121–3126. doi: 10.1161/STROKEAHA.107.493593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Group SR. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SPRINT MIND Investigators for the SPRINT Research Group, Williamson JD, Pajewski NM, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019;321(6):553–561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung AK, Rahman M, Reboussin DM, et al. Effects of Intensive BP Control in CKD. J Am Soc Nephrol 2017;28(9):2812–2823. doi: 10.1681/ASN.2017020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pajewski NM, Williamson JD, Applegate WB, et al. Characterizing frailty status in the systolic blood pressure intervention trial. J Gerontol Ser Biomed Sci Med Sci 2016;71(5):649–655. doi: 10.1093/Gerona/glv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 27.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged≥ 75 years: a randomized clinical trial. Jama 2016;315(24):2673–2682. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Julayanont P, Tangwongchai S, Hemrungrojn S, et al. The montreal cognitive assessment—basic: a screening tool for mild cognitive impairment in illiterate and low-educated elderly adults. J Am Geriatr Soc 2015;63(12):2550–2554.doi: 10.1111/jgs.13820 [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002;32(9):509–515. doi: 10.3928/0048-5713-20020901-06 [DOI] [Google Scholar]
- 30.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation 2010;121(11):1295–1303. doi: 10.1161/CIRCULATIONAHA.109.924266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bansal N, Katz R, Robinson-Cohen C, et al. Absolute Rates of Heart Failure, Coronary Heart Disease, and Stroke in Chronic Kidney Disease: An Analysis of 3 Community-Based Cohort Studies. JAMA Cardiol 2017;2(3):314–318. doi: 10.1001/jamacardio.2016.4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heung M, Steffick DE, Zivin K, et al. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. Am J Kidney Dis 2016;67(5):742–752. doi: 10.1053/j.ajkd.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004;15(5):1307–1315. doi: 10.1097/01.ASN.0000123691.46138.E2 [DOI] [PubMed] [Google Scholar]
- 34.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis 2004;43(5):861–867.doi: 10.1053/j.ajkd.2003.12.049 [DOI] [PubMed] [Google Scholar]
- 35.Chang C-C, Hsu C-Y, Chang T-Y, et al. Association between low-grade albuminuria and frailty among community-dwelling middle-aged and older people: a cross-sectional analysis from I-Lan Longitudinal Aging Study. Sci Rep 2016;6(1):1–9. doi: 10.1038/srep39434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darsie B, Shlipak MG, Sarnak MJ, Katz R, Fitzpatrick AL, Odden MC. Kidney Function and Cognitive Health in Older Adults: The Cardiovascular Health Study. Am J Epidemiol 2014;180(1):68–75. doi: 10.1093/aje/kwu102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurella M, Chertow GM, Fried LF, et al. Chronic Kidney Disease and Cognitive Impairment in the Elderly: The Health, Aging, and Body Composition Study. J Am Soc Nephrol 2005;16(7):2127–2133. doi: 10.1681/ASN.2005010005 [DOI] [PubMed] [Google Scholar]
- 38.Scheppach JB, Coresh J, Wu A, Gottesman RF, Mosley TH, Knopman DS, Grams ME, Sharrett AR, Koton S. Albuminuria and Estimated GFR as Risk Factors for Dementia in Midlife and Older Age: Findings From the ARIC Study. Am J Kidney Dis 2020December;76(6):775–783. doi: 10.1053/j.ajkd.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zijlstra LE, Trompet S, Mooijaart SP, et al. The association of kidney function and cognitive decline in older patients at risk of cardiovascular disease: a longitudinal data analysis. BMC Nephrol 2020;21(1):81. doi: 10.1186/s12882-020-01745-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikram MA, van Oijen M, de Jong FJ, et al. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke 2008;39(5):1421–1426. doi: 10.1161/STROKEAHA.107.501106 [DOI] [PubMed] [Google Scholar]
- 41.Yakushiji Y, Nanri Y, Hirotsu T, et al. Marked cerebral atrophy is correlated with kidney dysfunction in nondisabled adults. Hypertens Res 2010;33(12):1232–1237. doi: 10.1038/hr.2010.171 [DOI] [PubMed] [Google Scholar]
- 42.Kostapanos MS, Milionis HJ, Gazi I, Kostara C, Bairaktari ET, Elisaf M. Rosuvastatin Increases α−1 Microglobulin Urinary Excretion in Patients With Primary Dyslipidemia. J Clin Pharmacol 2006;46(11):1337–1343. doi: 10.1177/0091270006292629 [DOI] [PubMed] [Google Scholar]
- 43.Zhang WR, Parikh CR. Biomarkers of Acute and Chronic Kidney Disease. Annu Rev Physiol 2019;81:309–333. doi: 10.1146/annurev-physiol-020518-114605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol-Ren Physiol 2008;294(4):F697–F701. doi: 10.1152/ajprenal.00016.2008 [DOI] [PubMed] [Google Scholar]
- 45.Nadkarni GN, Rao V, Ismail-Beigi F, et al. Association of urinary biomarkers of inflammation, injury, and fibrosis with renal function decline: the ACCORD trial. Clin J Am Soc Nephrol 2016;11(8):1343–1352. doi: 10.2215/CJN.12051115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park M, Katz R, Shlipak MG, et al. Urinary markers of fibrosis and risk of cardiovascular events and death in kidney transplant recipients: the FAVORIT trial. Am J Transplant 2017;17(10):2640–2649. doi: 10.1111/ajt.14284 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


