Figure 3:
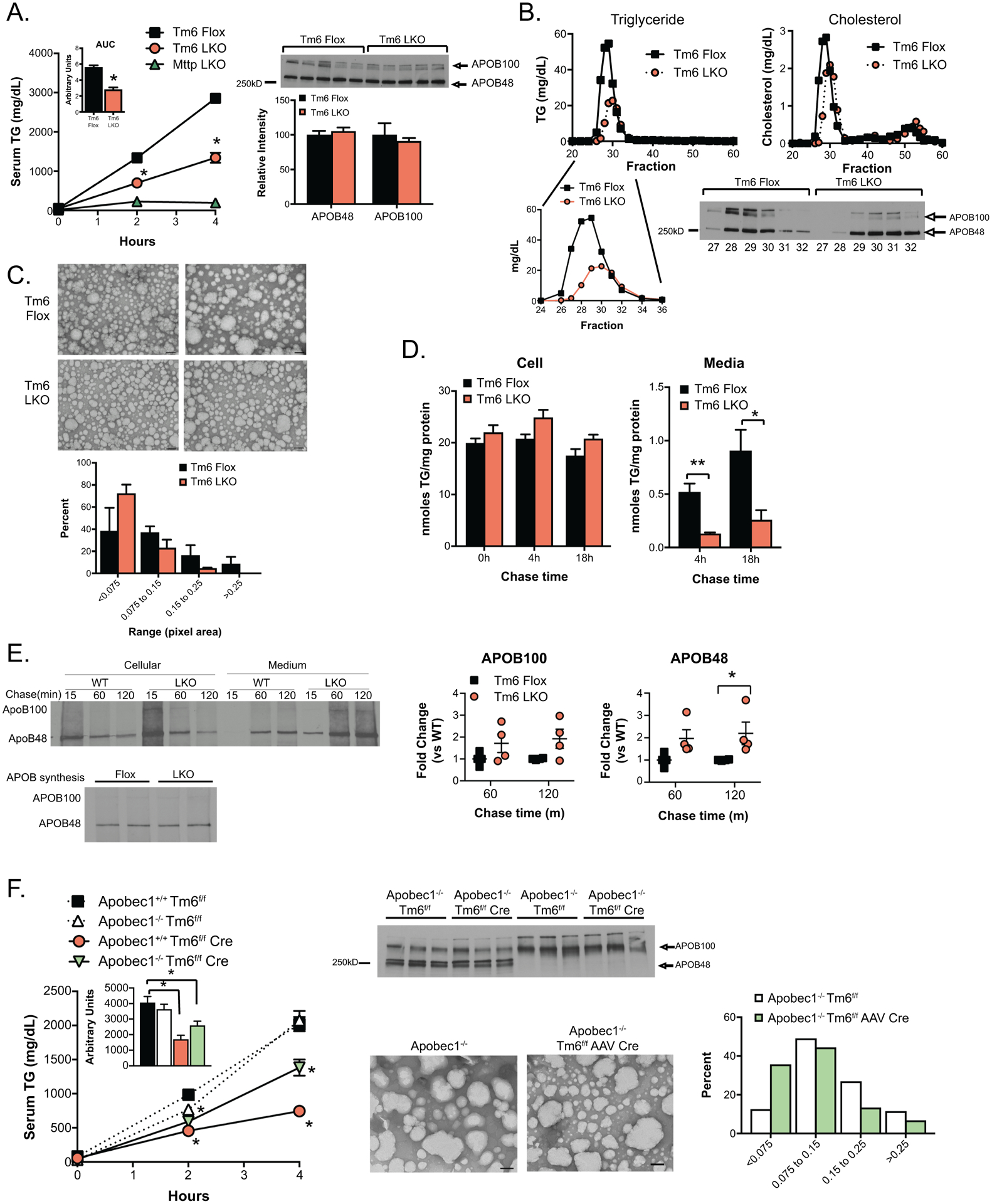
VLDL secretion characteristics of Tm6 LKO mice. A. Serum triglyceride levels at 0, 2 and 4 hours after injection of Pluronic −127 in Tm6 Flox, Tm6 LKO, and Mttp LKO mice (n=4–5/genotype). Inset shows average area under curve for Tm6 Flox and LKO genotypes. Right: Western blot of APOB protein in 4h serum of individual Tm6 Flox and Tm6 LKO mice, with densitometric quantification shown below. B. Serum from 4h bleed (Panel A) was pooled by genotype and fractionated by FPLC to separate lipoprotein particles. Column fractions were assayed for triglyceride and cholesterol to identify VLDL- and HDL-containing fractions (Fractions 27–32 and 48–55, respectively). Lower panel: Western blot showing APOB100 and APOB48 protein in VLDL FPLC fractions. C. Size distribution of negatively stained VLDL particles isolated from Tm6 Flox and Tm6 LKO mice. Serum was pooled from 3–4 mice per genotype 4h after Pluronic injection and fractionated by density centrifugation. Data are presented as percent of total droplets in each size range and was generated from 2 independent isolations per genotype. Representative images of negatively stained VLDL particles are shown (50,000x; scale bar=100nm). D. Synthesis and secretion of [3H]-labeled triglyceride in isolated primary hepatocytes. Left: Cellular [3H]-TG levels in Tm6 Flox and LKO hepatocytes at 0h, 4h and 18 hours after labeling, normalized to cellular protein. Right: [3H]-TG in media collected 4 or 18 hours after labeling. E. Synthesis and secretion of APOB in primary hepatocytes. Top left: Newly synthesized APOB100 and APOB48 in cells or medium 15, 60 or 120 minutes after pulse labeling of Tm6 Flox (WT) and LKO hepatocytes. Bottom left: APOB synthesis in isolated hepatocytes. Right: Quantitation of APOB100 and APOB48 secretion in 3 independent experiments, normalized to APOB secretion in WT hepatocytes in each experiment. F. Effect of Tm6sf2 deletion on VLDL secretion and particle size in APOB100 and APOB48 mice. Left: Serum triglyceride in HMFD-fed Apobec1+/+ Tm6f/f, Apobec1−/- Tm6f/f, Apobec1+/+ Tm6 f/f Cre and Apobec1−/- Tm6f/f Cre mice at 0, 2 and 4 hours after Pluronic F-127 injection. Deletion of Tm6sf2 was induced by AAV Cre in Apobec1−/- mice and with Alb CreTg in Apobec1+/+ mice. Tm6f/f mice received control AAV (null or LacZ). N=4–5 animals/group, mixed genders. Middle: Serum from 4 hour timepoint was analyzed by western blot analysis to monitor levels of APOB100 and APOB48 protein, and by density centrifugation to examine VLDL particle size in Apobec1−/- Tm6f/f and Apobec1−/- Tm6f/f AAV Cre mice fed chow diet. Representative images of negatively stained VLDL particles are shown (50,000x, scale bar=100nm), with quantitative size distribution presented as percent of total droplets in each size range (right). For all panels, asterisks indicate p<0.05 versus Tm6 Flox controls.
