Abstract
Background & Aims:
Achalasia is a debilitating chronic condition of the esophagus. Currently there are no national estimates on the epidemiologic and economic burden of disease. We sought to estimate trends in incidence and prevalence of achalasia by age-sex strata, and to estimate the total direct medical costs attributed to achalasia in the United States (U.S.).
Methods:
We conducted a cohort study using two administrative claims databases: IBM MarketScan Commercial Claims and Encounters database (2001–2018; age <65) and a 20% sample of nationwide Medicare enrollment and claims (2007–2015; age ≥65). Point prevalence was calculated on the first day of each calendar year; the incidence rate captured new cases developed in the ensuing year. Utilization rates of healthcare services and procedures were reported. Mean costs per patient were calculated and standardized to the corresponding U.S. Census Bureau population data to derive achalasia-specific total direct medical costs.
Results:
The crude prevalence of achalasia per 100,000 persons was 18.0 (95% CI: 17.4, 18.7) in MarketScan and 162.1 (95% CI: 157.6, 166.6) in Medicare. The crude incidence rate per 100,000 person-years was 10.5 (95% CI: 9.9, 11.1) in MarketScan and 26.0 (95% CI: 24.9, 27.2) in Medicare. Incidence and prevalence increased substantially over time in the Medicare cohort, and increased with more advanced age in both cohorts. Utilization of achalasia-specific healthcare was high; national estimates of total direct medical costs exceeded $408 million in 2018.
Conclusions:
Achalasia has a higher epidemiologic and economic burden in the U.S. than previously suggested, with diagnosis particularly increasing in older patients.
Keywords: achalasia, epidemiology, incidence, prevalence, cost
INTRODUCTION
Achalasia is a debilitating chronic condition of the esophagus that causes considerable morbidity for patients and warrants clinical intervention. The hallmark features of achalasia are esophageal aperistalsis and failure of the lower esophageal sphincter (LES) to relax.1−3 Symptoms include dysphagia, regurgitation, heartburn, chest pain, cough, and malnutrition.4 Achalasia negatively impacts quality of life and productivity.2,5 Additionally, compared to the general population, achalasia patients have an increased risk of lower respiratory tract infection, esophageal malignancy, and mortality.6,7 Treatment options include pro-motility agents, botulinum toxin injection, pneumatic dilation, Heller myotomy, and peroral endoscopic myotomy (POEM).8−17
The annual incidence and prevalence of achalasia have been estimated at 2 to 5 in 100,000 people and 11–32 per 100,000 people, respectively.18,19 However, these estimates have limitations. They come from older data (1996–2007)19, describe populations outside the U.S. or narrowly defined within the U.S, and do not provide age-sex-stratum specific measures of incidence and prevalence. There are no existing estimates on utilization of healthcare or treatment, nor national cost figures. Thus, there is a need for updated U.S. national estimates that present tailored statistics based on demographic factors such as age and sex, as well as an assessment in trends over time to examine how the national burden of disease may be shifting.
We aimed to estimate prevalence, incidence, utilization of treatments and health care services, and achalasia-associated costs by conducting a burden of disease study using administrative claims data from two U.S. populations. The epidemiologic estimates will allow clinicians and policymakers to understand how the burden of disease is changing nationally with shifting demographics, while stratified estimates will provide insight into subgroup differences in disease burden. Additionally, contemporary population-level cost and utilization estimates will help payers and providers allocate resources.
METHODS
Data source and study design
Two U.S. administrative claims databases were used to conduct a burden of disease analysis: MarketScan Commercial Claims and Encounters Database (Copyright © 2019 IBM Watson Health. All Rights Reserved.) and a 20% random sample from the Medicare program. MarketScan contains data on adults with commercial, employer-sponsored insurance and their dependents.20,21 Medicare enrollment and fee-for-service claims contain data on specific Medicare-enrolled beneficiaries, which include older Americans (age 65+) and those qualifying due to disability or end-stage-renal-disease. We employed a cohort study design to estimate annual measurements of prevalence, incidence, utilization, and costs from 2000–2018 (MarketScan) and 2008–2015 (Medicare). Data from 2000–2018 were used in MarketScan to determine long-term trends. Analysis for Medicare started in 2008 to allow for prescription drug data to be consistently populated (Part D drug coverage began in 2006).
Study population
We included all individuals younger than 65 in the MarketScan source population and adults age 65 and older in the Medicare source population. While adults age 65 and older with private insurance are contained in the MarketScan database, they were excluded from this analysis because they the comprehensiveness of their data is not guaranteed since they may have private insurance as a supplement to Medicare and the two data sources cannot be linked.
Prevalence and incidence definitions
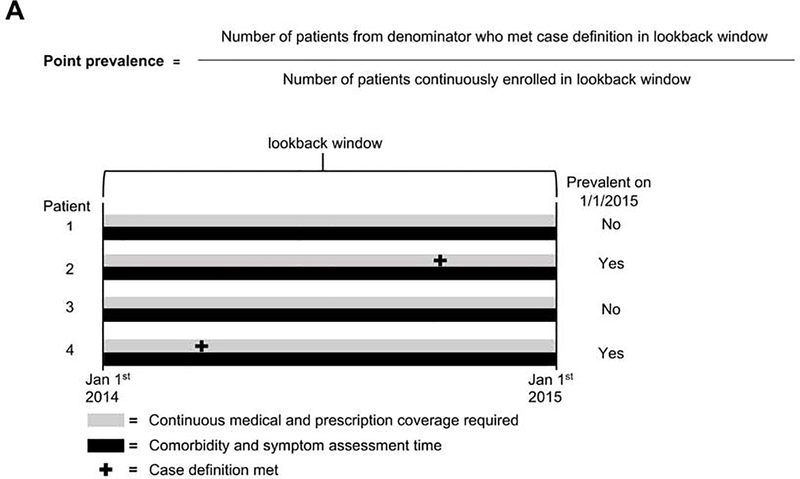
Annual point prevalence and incidence rate were calculated using an accepted methodology for estimating these parameters in administrative claims databases.22 Point prevalence describes the proportion of enrollees believed to currently have achalasia at a given time point (ex. January 1, 2015). Point prevalence was calculated as the proportion of enrollees with continuous enrollment in the lookback window (prior calendar year) who had at least one claim with an ICD-9-CM or ICD-10-CM diagnosis code (in any claim code position) for achalasia during the lookback window (530.0 or K22.0, respectively) (Figure 1). While there are no existing validation studies of claims-based algorithms for identifying achalasia cases, a prior study using MarketScan data used the presence of a single diagnosis code to identify a cohort of incident cases that went on to receive treatment.23 However, we performed sensitivity analyses around this case definition to provide a potential range of estimates (detailed below).
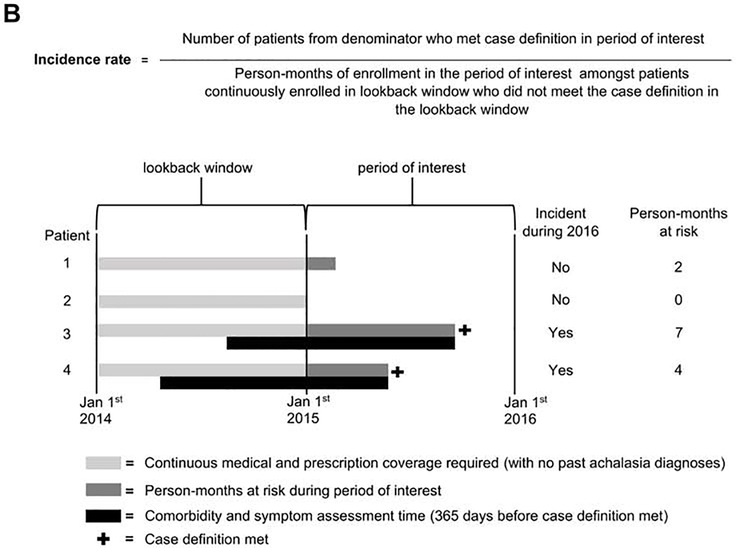
Figure 1.
Equations and study schematics for A) point prevalence and B) incidence rate
The incidence rate was calculated annually. The numerator was the number of enrollees who were continuously enrolled during the lookback window (prior calendar year) who had at least one claim with an ICD-9 (530.0) or ICD-10 (K220) diagnosis code (in any code position) for achalasia in the period of interest (e.g. 2015) but not in the lookback window (e.g. 2014). Thus, new achalasia cases were identified amongst a pool of at-risk individuals. The denominator was the sum of enrolled person-days in the analysis year amongst the at-risk pool. Person-days terminated at the first of: meeting the case definition, disenrolling from the insurance plan, dying (Medicare only), or reaching the end of that calendar year (Figure 1).
Prevalence and incidence were reported per 100,000 persons (person-years for incidence), with estimates calculated in aggregate and by age-sex strata (MarketScan: men <25, men 25–44, men 45–64, women <25, women 25–44, and women 45–64; Medicare: men 65–74, men 75–84, men ≥85, women 65–74, women 75–84, and women ≥85). When presenting the patient characteristics of incident and prevalent cases in the most recent year of data (2018 for MarketScan, 2015 for Medicare), such as comorbidities, a one-year covariate assessment window was used. We selected comorbidities based either on achalasia complications (candidal esophagitis; esophageal cancer) or potentially associated conditions. We also calculated an overall combined comorbidity score.24 Patient frailty was characterized using the Kim claims-based frailty index.25
We estimated national counts of combined prevalent cases and incident cases (“period prevalent cases”) in 2018. These were calculated by applying the most recent (2018 for MarketScan, 2015 for Medicare) age-sex-specific prevalence and incidence rates described above from both databases to corresponding national age-sex-specific population sizes in 2018 supplied by publicly available U.S. census data. The age-sex-specific prevalence and incidence rates for individuals <65 years of age came from MarketScan and those >65 from Medicare.
Utilization and Costs
Utilization rates of diagnostic procedures, treatment procedures, dispensed outpatient medications, and health care contacts were calculated in the total population of period prevalent patients. For prevalent patients, follow-up began on January 1st of the analysis year. For incident patients, follow-up began at first diagnosis. In calculating rates, the numerator was the number of procedures or prescriptions and the denominator was person-time enrolled in the calendar year as a known achalasia case (existing or new). Codes used to identify procedures and medications of interest are specified in the supplement.
A national estimate of direct annual non-prescription medical costs attributed to achalasia in 2018 was calculated in a three-step process using age-sex-specific mean costs from both databases, estimates of prevalence and incidence, and population data from the U.S. census. Further details are provided in the Supplement eTable 2.
Statistical analyses
Temporal trends in prevalence and incidence rate were assessed using multivariable Poisson regression models adjusted for age-sex stratum, year of diagnosis, and interaction terms between age-sex stratum and time. These models were used to explore trends in prevalence and incidence by age and sex subgroup. All analyses were performed using SAS 9.4 (Cary, NC). Annual percent change (APC) was reported for utilization trends by the following formula:
Where βtime was the coefficient from a linear term for year of diagnosis in the model.
Sensitivity analyses
The primary case definition could provide overestimates, as it emphasizes sensitivity (fewer false negatives) by only requiring one inpatient or outpatient diagnosis code. As a sensitivity analysis, the presence of one inpatient diagnosis code or two outpatient diagnosis codes was used as an alternative case definition, representing a potentially more specific (fewer false positives) assessment. An additional layer of sensitivity analyses was applied, restricting the primary case definition and definitions above to those with a primary diagnosis code of achalasia instead of allowing any diagnosis position.
RESULTS
Study population
In the MarketScan cohort during 2018, we identified 2,900 prevalent patients on January 1st, and 1,272 patients who developed incident achalasia during the ensuing year (Table 1). The median age of prevalent cases was 52.7 years and 56% were female. The most diagnosed symptoms in prevalent cases were dysphagia (41.1%) and esophageal reflux/heartburn (54.0%). Nearly three-quarters of cases were in the robust category of a claims-based frailty index. In the Medicare cohort during 2015, we identified 4,907 prevalent patients and 2,051 incident patients (Table 1). The median age of prevalent cases was 78.0 and 62.7% were female. Common symptoms (prevalent cases) included dysphagia (19.4%), reflux/heartburn (61.0%), and pneumonia (17.5%). Over 32% of prevalent cases were categorized as mildly frail or moderately-to-severely frail using the claims-based Kim frailty index.
Table 1.
Demographic and clinical characteristics of prevalent and incident achalasia patients using the latest year of data in MarketScan (2018) and Medicare Databases (2015).
| MarketScan |
Medicare |
|||
|---|---|---|---|---|
| Prevalent patients N= 2,900 | Incident patients N= 1,272 | Prevalent patients N= 4,907 | Incident patients N= 2,051 | |
| Age, median (IQR) | 52.7 (41.4–59.3) | 52.6 (41.5–59.7) | 78.0 (72.0–84.5) | 78.1 (72.2–84.6) |
| Age, n (%) | ||||
| 0–17 | 90 (3.1) | 42 (3.3) | -- | -- |
| 18–24 | 133 (4.6) | 70 (5.5) | -- | -- |
| 25–34 | 247 (8.5) | 113 (8.9) | -- | -- |
| 35–44 | 446 (15.4) | 183 (14.4) | -- | -- |
| 45–54 | 792 (27.3) | 327 (25.7) | -- | -- |
| 55–64 | 1192 (41.1) | 537 (42.2) | -- | -- |
| 65–74 | -- | -- | 1,958 (34.9) | 794 (38.7) |
| 75–84 | -- | -- | 1,877 (38.3) | 804 (39.2) |
| ≥85 | -- | -- | 1,072 (21.9) | 453 (22.1) |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic White | -- | -- | 4,360 (89.3) | 1,821 (89.1) |
| Non-Hispanic Black | -- | -- | 341 (7.0) | 142 (7.0) |
| Non-Hispanic Asian | -- | -- | 51 (1.0) | 25 (1.2) |
| Non-Hispanic North Native American | -- | -- | 23 (0.5) | * |
| Hispanic | -- | -- | 64 (1.3) | 24 (1.2) |
| Non-Hispanic Other | -- | -- | 44 (0.9) | 20 (1.0) |
| Unknown | -- | -- | 24 | * |
| Sex, n (%) | ||||
| Male | 1,276 (44.0) | 550 (43.2) | 1,830 (37.3) | 802 (39.1) |
| Female | 1,624 (56.0) | 722 (56.8) | 3,077 (62.7) | 1,249 (60.9) |
| Symptomsa,b, n (%) | ||||
| Dysphagia | 1,192 (41.1) | 705 (55.4) | 953 (19.4) | 421 (20.5) |
| Esophageal reflux and heartburn | 1,566 (54.0) | 807 (63.4) | 2,992 (61.0) | 1,295 (63.1) |
| Chest pain | 665 (22.9) | 325(25.6) | 784 (16.0) | 377 (13.4) |
| Weight loss | 189 (6.5) | 101 (7.9) | 648 (13.2) | 290 (14.1) |
| Ulcers and esophageal bleeding | 122 (4.2) | 79 (6.2) | 18 (0.4) | * (<0.6) |
| Pneumonia | 177 (6.1) | 80 (6.2) | 860 (17.5) | 408 (19.9) |
| Select comorbiditiesa,b, n (%) | ||||
| Barrett’s Esophagus | 197 (6.8) | 92 (7.2) | 270 (5.5) | 116 (5.7) |
| Candidal esophagitis | 51 (1.8) | 31 (2.4) | 142 (2.9) | 52 (2.5) |
| Anemia | 392 (13.5) | 179 (14.1) | 1,968 (40.1) | 861 (42.0) |
| Esophageal cancer | 15 (0.5) | 10 (0.8) | 47 (1.0) | 19 (1.0) |
| Other gastrointestinal cancers | 31 (1.1) | 16 (1.3) | 190 (3.9) | 93 (4.5) |
| Asthma and COPD | 416 (14.3) | 182 (14.3) | 1,686 (34.4) | 725 (35.4) |
| Rheumatoid arthritis | 66 (2.3) | 25 (2.0) | 286 (5.8) | 113 (5.5) |
| Scleroderma or systemic sclerosis | 38 (1.3) | 17 (1.3) | 75 (1.5) | 29 (1.4) |
| Lupus | 30 (1.0) | 13 (1.0) | 55 (1.1) | 22 (1.1) |
| Psoriatic arthritis | 19 (0.7) | 7 (0.6) | 23 (0.5) | 12 (0.6) |
| Sicca syndrome | 29 (1.0) | 13 (1.0) | 67 (1.4) | 28 (1.4) |
| Sarcoidosis | 19 (0.7) | 10 (0.8) | 19 (0.4) | * (<0.6) |
| Multiple sclerosis | 16 (0.6) | 9 (0.7) | 28 (0.6) | * (<0.6) |
| Ulcerative colitis | 37 (1.3) | 13 (1.0) | 55 (1.1) | 23 (1.1) |
| Crohn’s disease | 22 (0.8) | 10 (0.8) | 43 (0.9) | 23 (1.1) |
| Gagne comorbidity scorea,b, n (%) | ||||
| −1 | 335 (11.6) | 125 (9.8) | 470 (9.6) | 149 (7.2) |
| 0 | 1,364 (47.0) | 835 (65.6) | 886 (18.1) | 321 (15.7) |
| 1 | 608 (21.0) | 190 (14.9) | 764 (15.6) | 307 (15.0) |
| 2 | 251 (8.7) | 60 (4.7) | 569 (11.6) | 243 (11.9) |
| >3 | 342 (11.8) | 62 (4.9) | 2,218 (45.2) | 1,031 (50.3) |
| Kim Frailty Indexa,b, n (%) | ||||
| Robust, <0.15 | 2,156 (74.3) | 937 (73.7) | 1,178 (24.0) | 419 (20.4) |
| Prefrail, 0.15–0.24 | 680 (23.5) | 307 (24.1) | 2,130 (43.4) | 903 (44.0) |
| Mildly frail, 0.25–0.34 | 59 (2.0) | 27 (2.1) | 1,077 (22.0) | 507 (24.7) |
| Moderate-to-severely frail, ≥0.35 | 5 (0.2) | 1 (0.1) | 522 (10.6) | 222 (10.8) |
For incident cases, one-year of prior continuous insurance enrollment before index diagnosis was required and served as the lookback window to assess the presence of diagnostic codes that indicated the specified symptoms and comorbidities.
For prevalent cases, a one-year lookback window was used from the last date of enrollment or the end of the calendar year (whichever came first) to assess the presence of diagnostic codes that indicated the specified symptoms and comorbidities.
Cell counts less than 11 are suppressed per CMS cell size suppression policy
Prevalence and Incidence
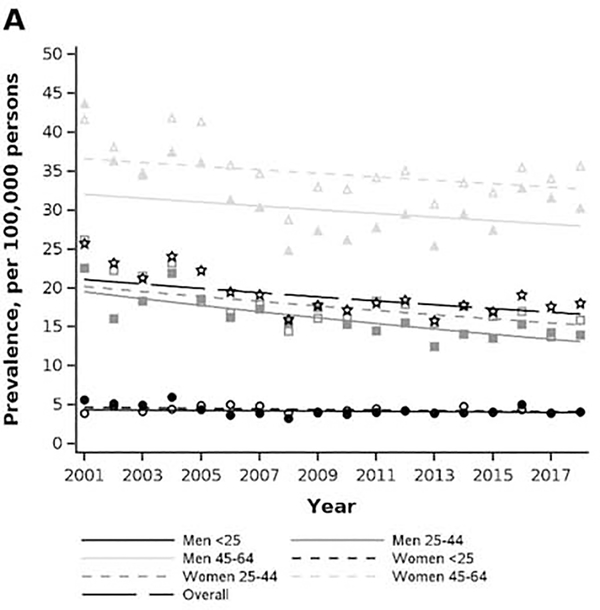
The crude prevalence of achalasia in the MarketScan cohort was 18.0 per 100,000 (95% CI: 17.4, 18.7) in 2018, compared to 25.7 per 100,000 (95% CI: 23.3, 28.2) in 2001 (Figure 2A). Overall, the prevalence increased with older age and was highest in women aged 45–64 (2018 estimate: 35.6 per 100,000, 95% CI: 33.6, 37.7). Women had a higher prevalence of achalasia than men in the two older age-strata, but differences by sex were negligible in the <25 age stratum. In terms of age-sex stratum-specific temporal trends, the prevalence was stable in both men and women <25 and decreased in all other strata. The decrease was sharpest in men 25–44, with a −2.3% (95% CI: 1.7%, 2.9%) annual percent change in prevalence from 2001–2018.
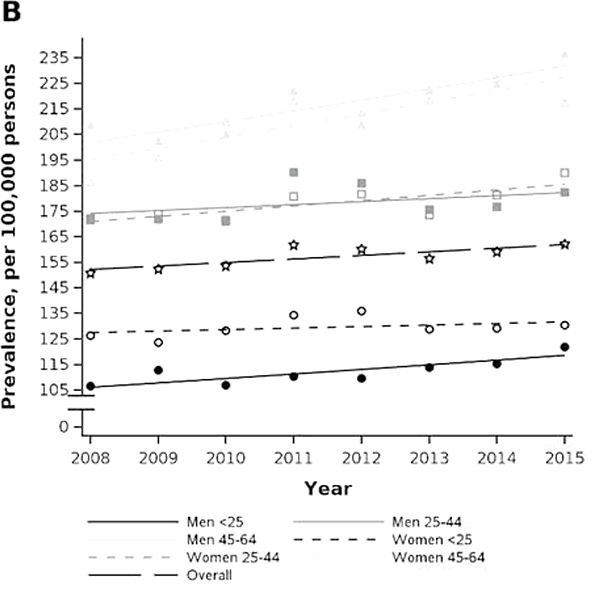
Figure 2.
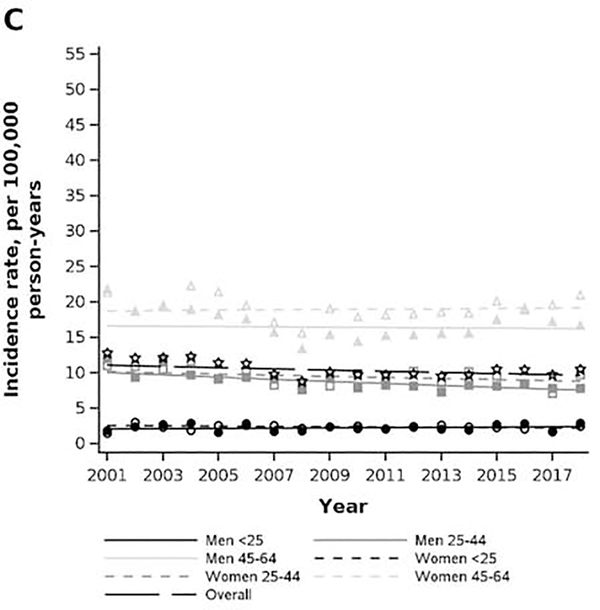
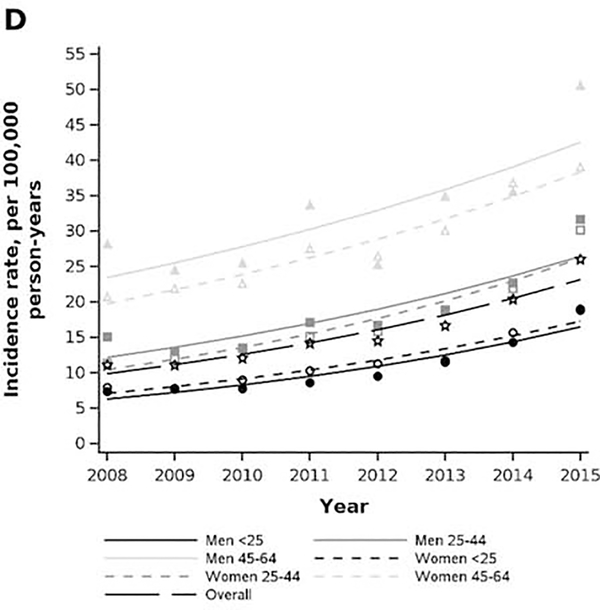
Age and sex stratum-specific trends in prevalence and incidence rate of achalasia in privately insured (2001–2018) and Medicare-insured (2008–2015) populations. A) MarketScan prevalence. B) Medicare prevalence. C) MarketScan incidence rate. D) Medicare incidence rate.
The crude prevalence of achalasia in the Medicare cohort was 162.1 per 100,000 individuals (95% CI: 157.6, 166.6) in 2015, which was an increase since 2001 when the prevalence was 150.7 (95% CI: 145.6, 155.9) (Figure 2B). The prevalence among older adults also increased with older age and was highest amongst men 85 and older at (2018 estimates: 236.8 per 100,000, 95% CI: 210.9, 262.6). Women 85 and older had the greatest annual percent change in prevalence, increasing at 2.2% (95% CI: 1.0, 3.4) from the prior year across 2007–2015.
In the MarketScan cohort, the crude incidence rate of achalasia was 10.5 per 100,000 person-years (95% CI: 9.9, 11.1) in 2018, a slight decrease from an incidence rate of 12.8 per 100,000 person-years (95% CI: 11.0, 14.8) in 2001 (Figure 2C). The incidence rate increased with older age and was highest in women aged 45–64 at (2018 estimate: 21.0, 95% CI: 19.2, 22.9) per 100,000 person-years. The incidence-rate was largely stable over time for all age-sex strata, except for a slight decrease in the stratum of men aged 25–44, where the incidence rate had an average percent change of −1.7% (95% CI: −2.6%, −0.7%).
In the Medicare cohort, the crude incidence rate of achalasia was 26.0 per 100,000 person-years (95% CI: 24.9, 27.2) in 2015, an increase from an incidence rate of 11.1 (95% CI: 10.5, 11.7) in 2001 (Figure 2D). The incidence rate was highest in men 85+ (2015 estimate: 50.6 cases per 100,000-person-years, 95% CI: 43.1, 59.4) and lowest in women 65–74 (2015 estimate:18.8, 95% CI: 17.2, 20.6). Regarding temporal trends, the incidence-rate increased over time for all age-sex strata, with the steepest increase in men aged 65–74, who had an annual percent change in incidence rate of 14.8% (95% CI: 12.5, 17.1) from 2008–2015.
Using the most current age-sex-specific prevalence and incidence rate estimates from both databases, coupled with age-sex-specific 2018 U.S. census population size estimates, we estimated that in 2018 there were 166,223 patients with existing or new achalasia among the U.S. population.
Our sensitivity analyses demonstrated that estimates of incidence and prevalence changed depending on the case definition used (Supplement eFigure 1). For example, in Medicare, the estimated prevalence in 2015 dropped from about 160 cases per 100,000 using the primary definition to 40 cases per 100,000 using the most stringent definition which required either one inpatient or two outpatient diagnosis codes (on different dates) in the primary diagnosis position. Similarly, comparing these case definitions, the estimate of the incidence rate in Medicare decreased from about 25 to 4 per 100,000 person-years. In parallel, decreases were also observed in the MarketScan cohort when applying this more stringent definition, with the 2018 prevalence changing from about 17 to just under 4 per 100,000 and the incidence rate changing from about 10 to 1 per 100,000 person-years. While the actual values of the measures were sensitive to the case definition, the decreasing trends in MarketScan and increasing trends in Medicare were similar across definitions (supplemental materials).
Utilization
In both cohorts, utilization of achalasia-specific outpatient visits was high, with an estimated 1,535 and 629 outpatient visits per 1,000 person-years in the MarketScan and Medicare cohorts, respectively (Tables 2 and 3). Hospitalizations for achalasia decreased in the MarketScan cohort (APC −3.5, 95% CI: −5.2, −1.9), but remained steady in the Medicare cohort (APC 0.2, 95% CI: −1.4, 1.8). Other notable trends included an increase in reflux monitoring, as well as unlisted procedures of the esophagus, a CPT code that may have been used to document peroral endoscopic myotomy (POEM). The use of promotility drugs declined substantially over the years. Esophagectomy was rarely performed.
Table 2.
Temporal trends in healthcare utilization rates of period-prevalent patients per 1000 enrolled person-years, by study year in MarketScan.
| Period prevalent patients, N |
2011 4,908 |
2012 5,921 |
2013 4,386 |
2014 4,854 |
2015 4,467 |
2016 4,514 |
2017 3,892 |
2018 3,854 |
APC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Healthcare contacts* per 1000 enrollee years | |||||||||
| Hospitalizations | 94.2 | 97.5 | 101.2 | 95.4 | 87.8 | 84.8 | 86.0 | 70.4 | −3.5 (−5.2, −1.9) |
| Emergency room visits | 35.7 | 40.6 | 45.8 | 52.3 | 45.3 | 50.7 | 38.3 | 40.7 | 1.3 (−1.1, 3.8) |
| Outpatient visits | 1,263.5 | 1,357.6 | 1,400.4 | 1,455.9 | 1,493.7 | 1,590.8 | 1,584.6 | 1,535.4 | 3.1 (2.7, 3.5) |
| Diagnostic procedures per 1000 enrollee years | |||||||||
| Thorax CT or X-ray | 614.3 | 704.9 | 665.2 | 684.1 | 635.3 | 743.6 | 755.6 | 655.9 | 1.3 (0.6, 1.9) |
| Barium swallow | 326.4 | 392.5 | 357.0 | 344.4 | 324.1 | 397.3 | 367.9 | 353.1 | 0.4 (−0.4, 1.3) |
| Esophagoscopy & UE | 743.3 | 789.0 | 785.1 | 729.8 | 757.6 | 852.0 | 852.0 | 904.6 | 2.4 (1.8, 3.0) |
| Manometry | 158.3 | 182.5 | 173.3 | 185.1 | 201.5 | 210.8 | 230.9 | 218.1 | 4.9 (3.7, 6.1) |
| Reflux monitoring | 69.5 | 70.4 | 90.5 | 84.9 | 102.3 | 116.5 | 121.8 | 120.9 | 9.4 (7.7, 11.3) |
| Therapeutic procedures per 1000 enrollee years | |||||||||
| Pneumatic dilation | 16.7 | 29.0 | 16.1 | 18.7 | 26.2 | 28.8 | 16.8 | 30.6 | 3.9 (0.5, 7.4) |
| Surgical myotomy | 92.9 | 106.7 | 103.8 | 98.6 | 87.5 | 103.0 | 101.3 | 87.0 | −1.0 (−2.6, 0.6) |
| Anti-reflux surgery | 111.9 | 119.9 | 126.6 | 120.4 | 108.3 | 131.6 | 127.3 | 111.5 | 0.5 (−1.0, 1.9) |
| Esophagectomy | 5.1 | 4.1 | 4.3 | 6.0 | 4.8 | 6.7 | 3.2 | 2.3 | −3.6 (−10.6, 3.9) |
| Unlisted procedure of esophagus† | 0.5 | 5.8 | 5.2 | 12.1 | 20.0 | 16.0 | 19.5 | 31.6 | 36.5 (30.0, 43.4) |
| Dispensed medications, prescriptions per 1000 enrollee years | |||||||||
| CCBs | 292.0 | 318.4 | 372.2 | 370.9 | 423.8 | 521.9 | 547.8 | 504.3 | 9.6 (8.7, 10.5) |
| PPIs | 918.3 | 1,232.8 | 1,393.0 | 1,246.4 | 1,516.2 | 1,788.5 | 1,708.6 | 1,693.9 | 8.2 (7.7, 8.7) |
| Nitrates | 20.3 | 22.6 | 38.1 | 32.9 | 41.6 | 47.6 | 72.4 | 88.7 | 23.3 (20.2, 26.4) |
| Anticholinergics | 65.7 | 40.6 | 68.0 | 68.1 | 78.9 | 64.4 | 65.9 | 57.4 | 2.0 (0.0, 4.1) |
| Antidepressants & neuromodulators | 935.3 | 1,012.1 | 978.0 | 1,379.2 | 1,502.3 | 1,595.3 | 1,668.0 | 1,569.0 | 9.3 (8.9, 9.8) |
| Opioid medications | 583.0 | 590.1 | 698.3 | 768.2 | 939.4 | 926.2 | 948.5 | 769.3 | 6.7 (6.1, 7.3) |
| Pro-motility drugs | 59.5 | 45.5 | 27.7 | 30.2 | 31.9 | 42.6 | 20.1 | 21.2 | −11.4 (−13.8, −8.9) |
Abbreviations: GI, gastrointestinal; EKG, electrocardiogram; UE, upper endoscopy; CCBs, calcium channel blockers; PPIs, proton pump inhibitors
Hospitalizations, emergency department visits, and office visits all required a diagnosis of achalasia in the first or second diagnosis position on the claim Period prevalent includes patient with prevalent disease on January 1st of a given year and incident cases that develop achalasia in that calendar year.
Peroral endoscopic myotomy (POEM) does not have a specified CPT code, currently billed as unlisted procedure of the esophagus
Table 3.
Temporal trends in healthcare utilization rates of period-prevalent patients per 1000 enrolled person-years, by study year in Medicare
| Period prevalent patients, N |
2008
4,728 |
2009
4,776 |
2010
4,883 |
2011
5,318 |
2012
5,388 |
2013
5,635 |
2014
6,604 |
2015
6,958 |
APC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Healthcare services* per 1000 enrollee years | |||||||||
| Hospitalizations | 75.2 | 85.3 | 79.6 | 88.2 | 75.9 | 74.9 | 76.2 | 86.4 | 0.2 (−1.4, 1.8) |
| Emergency room visits | 17.3 | 12.4 | 10.8 | 12.7 | 11.8 | 14.2 | 13.5 | 18.6 | 2.8 (−1.1, 6.8) |
| Outpatient visits | 468.6 | 502.1 | 500.3 | 523.7 | 553.9 | 578.5 | 613.1 | 628.6 | 4.4 (3.7, 5.0) |
| Diagnostic procedures per 1000 enrollee years | |||||||||
| Thorax CT or X-ray | 785.2 | 867.8 | 781.4 | 809.9 | 819.9 | 844.3 | 838.1 | 849.1 | 0.7 (0.2, 1.3) |
| Barium swallow | 279.9 | 311.6 | 299.6 | 289.5 | 293.2 | 303.1 | 294.4 | 309.5 | 0.6 (−0.3, 1.4) |
| Esophagoscopy & UE | 847.9 | 907.6 | 840.4 | 870.4 | 833.5 | 872.0 | 849.2 | 835.9 | −0.5 (−0.9, 0.0) |
| Manometry | 90.9 | 92.4 | 90.4 | 99.7 | 98.1 | 113.3 | 122.0 | 132.5 | 6.1 (4.7, 7.6) |
| Reflux monitoring | 18.6 | 20.5 | 23.1 | 25.4 | 37.2 | 48.8 | 54.6 | 59.9 | 20.6 (17.6, 23.7) |
| Therapeutic procedures per 1000 enrollee years | |||||||||
| Pneumatic dilation | 13.6 | 12.6 | 8.0 | 10.3 | 9.5 | 10.9 | 12.7 | 10.4 | −1.1 (−5.3, 3.2) |
| Surgical myotomy | 9.6 | 21.1 | 20.5 | 17.2 | 19.3 | 22.6 | 23.3 | 23.4 | 7.2 (3.7, 10.7) |
| Anti-reflux surgery | 15.7 | 29.7 | 27.0 | 32.9 | 32.2 | 38.1 | 35.0 | 39.4 | 8.7 (5.9, 11.5) |
| Esophagectomy | 1.9 | 3.2 | 2.1 | 2.4 | 1.8 | 2.2 | 1.7 | 1.4 | −6.1 (−15.0, 3.7) |
| Unlisted procedure of esophagus† | 3.7 | 2.9 | 2.8 | 4.2 | 6.8 | 5.5 | 5.3 | 11.3 | 19.7 (12.1, 27.8) |
| Dispensed medications, prescriptions per 1000 enrollee years | |||||||||
| CCBs | 900.8 | 867.8 | 948.2 | 1,081.8 | 1,133.3 | 1,191.3 | 1,123.3 | 1,144.6 | 4.1 (3.7, 4.6) |
| PPIs | 1,114.8 | 1,630.1 | 1,280.9 | 1,476.9 | 1,877.3 | 2,311.2 | 2,285.3 | 2,418.6 | 11.1 (10.8, 11.5) |
| Nitrates | 634.5 | 422.3 | 407.1 | 295.8 | 244.4 | 308.2 | 311.5 | 346.5 | −8.2 (−8.8, −7.5) |
| Anticholinergics | 115.6 | 74.7 | 31.6 | 4.7 | 1.1 | 6.9 | 9.5 | 5.9 | −43.5 (−45.7, −41.3) |
| Antidepressants & neuromodulators | 1,251.6 | 1,394.4 | 1,427.7 | 1,642.0 | 1,795.1 | 1,894.1 | 2,257.5 | 2,484.1 | 10.5 (10.1, 10.9) |
| Opioid medications | 582.4 | 582.6 | 701.0 | 849.9 | 905.7 | 1,057.8 | 1,222.1 | 1,313.0 | 13.5 (12.9, 14.0) |
| Pro-motility drugs | 302.7 | 180.3 | 115.3 | 134.0 | 76.3 | 104.4 | 87.1 | 45.6 | −19.9 (−21.0, −18.9) |
Abbreviations: GI, gastrointestinal; EKG, electrocardiogram; UE, upper endoscopy; CCBs, calcium channel blockers; PPIs, proton pump inhibitors
Hospitalizations, emergency department visits, and office visits all required a diagnosis of achalasia in the first or second diagnosis position Period prevalent includes patient with prevalent disease on January 1st of a given year and incident cases that develop achalasia in that calendar year.
Peroral endoscopic myotomy (POEM) does not have a specified CPT code, currently billed as unlisted procedure of the esophagus
Costs
Applying the stratum-specific mean costs we estimated in both databases to our national estimates of period-prevalent cases, we estimated that nationally there were $408,479,778 in direct medical costs for achalasia in 2018 (Table 4). Notably, when we restrict to only incident cases, the mean costs were higher, and was particularly noticeable in younger incident cases (Supplement eTable 1). For example, for a male <25 years with a prevalent case, annual average costs were $3,701.29, whereas costs for an incident case were $8,059.46.
Table 4.
National estimates of direct healthcare costs attributed to achalasia (prevalent and incident cases) in 2018.
| Calculated from sample Mean Mean inpatient outpatient costs costs |
||||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Age group | N | Mean inpatient costs | Mean outpatient costs | Mean Total costs | Census population size estimate | Estimated period prevalent cases | Total economic burden, dollars |
| Male | <25 | 153 | 3,921.58 | 2,074.20 | 5,995.78 | 53,239,029 | 3,701.29 | 22,192,109.69 |
| Male | 25–44 | 455 | 2,127.67 | 1,995.98 | 4,123.65 | 43,671,974 | 9,516.46 | 39,242,544.84 |
| Male | 45–64 | 1,326 | 1,852.86 | 1,583.79 | 3,436.66 | 40,916,250 | 19,214.10 | 66,032,331.41 |
| Male | 65–74 | 1,123 | 1,274.49 | 366.5 | 1,641.00 | 14,277,428 | 20,130.46 | 33,034,083.07 |
| Male | 75–84 | 1,037 | 748.89 | 461.39 | 1,210.28 | 6,787,377 | 14,534.84 | 17,591,232.15 |
| Male | ≥85 | 472 | 705.6 | 555.5 | 1,261.08 | 2,226,093 | 6,397.43 | 8,067,675.487 |
| Female | <25 | 148 | 3,695.38 | 2,267.01 | 5,962.39 | 50,769,563 | 3,278.33 | 19,546,660.59 |
| Female | 25–44 | 478 | 1,563.53 | 2,175.84 | 3,739.38 | 43,171,153 | 11,042.54 | 41,292,271.44 |
| Female | 45–64 | 1,657 | 1,361.52 | 1,068.51 | 2,848.09 | 42,976,356 | 24,335.92 | 69,310,901.56 |
| Female | 65–74 | 1,629 | 683.50 | 330.85 | 1,014.35 | 16,293,885 | 24,306.96 | 59,066,644.79 |
| Female | 75–84 | 1,644 | 786.62 | 344.07 | 1,130.68 | 8,760,576 | 19,299.36 | 21,821,395.67 |
| Female | ≥85 | 1,053 | 618.46 | 459.63 | 1,078.09 | 4,077,755 | 10,464.74 | 11,281,927.33 |
| TOTAL | 327,167,439 | 166,223 | 408,479,778.04 | |||||
Age-sex strata census population size estimate obtained from the United State Census Bureau 2018 American Community Survey obtained at https://data.census.gov/cedsci/table?q=United%20States&tid=ACSDP1Y2018.DP05&hidePreview=true
Stratum-specific incidence obtained from conversion of calculated stratum-specific incidence rate via the exponential formula
Estimated period prevalent cases = (stratum-specific prevalence)(census population size estimate) + (stratum-specific incidence)(census population size estimate)
Stratum total economic burden = (stratum-specific estimated period prevalent cases)(stratum-specific total costs)
DISCUSSION
Esophageal achalasia is a debilitating chronic disease that causes considerable morbidity and mortality, but the epidemiology had been incompletely described. In an examination of databases encompassing a large proportion of the population of the U.S., our study found a strikingly higher incidence and prevalence than prior literature suggested, particularly in older adults. Given that the prevalence in the Medicare population is an estimated 162 patients per 100,000, gastroenterologists are likely to come across this disease in clinical practice and should not necessarily view it as a very rare diagnosis. The high prevalence in the older age strata suggests that the increase in the crude prevalence over time is likely due to the aging of the U.S. population. As expected, the Medicare population had much higher comorbidity rates (ex. 35% of incident cases with asthma or COPD) than the younger MarketScan population (14% with asthma or COPD). The burden of concomitant conditions at or after diagnosis may have implications for managing the care of more medically complex patients. Given the observed increased incidence of achalasia with age, etiologic studies are warranted to determine whether these comorbidities may be risk factors or are similarly heightened with age in non-achalasia controls.
We found that achalasia-specific healthcare utilization was high in both cohorts, with a steady increase in the outpatient visit rate across the most recent 8 years of data. Although the nature of an “unlisted procedure of the esophagus” code is unknown, the precipitous increase in the utilization of this code does align with the introduction of POEM, which does not currently have a specific CPT code. We additionally found that achalasia patients had considerable medical costs (approaching a half billion dollars) and mean costs were heightened when considering a cohort restricted to incident patients, likely on account of up front clinical and surgical management of disease.
In comparison to our findings, existing studies on the incidence and prevalence of achalasia have found lower estimates of these population health parameters. A population-based study of Canadian administrative billing data from 1997–2007 found an incidence and prevalence of 1.63 per 100,000 and 10.82 per 100,000, respectively.19 These estimates may be lower than ours for several reasons. The study population is different geographically and temporally, and risk factors for achalasia may differ accordingly. Critically, the case definition was stricter by focusing on treated achalasia and requiring either pneumatic dilation or esophagectomy procedure codes to accompany the diagnosis code. This increased specificity (lower percentage of false positives) but decreased sensitivity (higher percentage of false negatives). However, even with our most stringent case definition sensitivity analysis, the overall prevalence is 3.1 per 100,000 in MarketScan and 45.4/100,000 in Medicare. Another study used institutional electronic health records to estimate the incidence and prevalence of achalasia in the Chicago area.18 The authors reported an incidence of 4.60 per 100,000 and a prevalence of 32.58 per 100,000. The strength of the study was the rigorous assessment of medical record data, with manual review of diagnostic test results and clinical notes. However, it is not known if the results generalize nationally, and the estimates assumed that all cases from the denominator of the selected geographic area would have—if they were a case—been seen at the institution from which the data were collected.
Existing studies have strength and limitation profiles that differ from our presented study, making our contribution complimentary to the existing epidemiologic literature. In contrast with electronic medical records from single health care provider system, we used administrative claims data, which capture data from across healthcare settings and over a broader population (not just one system). Given a patient has insurance enrollment, the entirety of the patient’s billed medical care will be captured in a claims database regardless of where the care is received. The central limitation of claims data is lack of clinical detail and the inability to assess rates in the uninsured. Our multi-database study is the first to report estimates of incidence, prevalence, and costs from two sources that both contain patients from across the nation. By using both MarketScan (40 million enrollees in database annually) and Medicare, we were able to capture a large proportion of insured individuals in the U.S.26 Our Medicare sample is highly representative of the older patient population, as nearly 70% of adults over the age of 65 are enrolled in Medicare Fee For Service.27
Limitations of our study include the lack of validated case algorithms. However, the symptoms we documented in Table 1 are consistent with achalasia and we also conducted a range of sensitivity analyses with more stringent case definitions. The estimates were noticeably smaller when implementing these case requirements, but they do not change the qualitative conclusion of the analysis that achalasia has a higher epidemiologic and economic burden than previously suggested, particularly in older adults. Additionally, to report one long-term summary trend metric, we assumed a constant annual percent change over study years. This may have smoothed over possible sub-trends marked by inflection points. For instance, the early years of the MarketScan data appear to show a sharper decline followed by a leveling. However, these years contribute fewer data and carry smaller weights in the calculation of the summary metric. They also are subject to more random error from smaller annual sample sizes.
In summary, achalasia has a higher incidence and prevalence in the United States than previously reported. Thus, achalasia should be on the gastroenterologist’s differential diagnosis for dysphagia and reflux patients, and the condition should be expected to be encountered in routine practice. Future research should estimate achalasia risk after dysphagia diagnosis. Our finding that incidence and prevalence increases with age calls into question whether older adults are more susceptible to this debilitating disease or what past exposures may contribute to this increased risk in later stages in the life course. The economic burden of disease was substantial, and coupled with the epidemiologic estimates, suggest that achalasia warrants increased research investment across the spectrum from etiologic research to comparative effectiveness assessments of existing and emerging treatments.
Supplementary Material
What You Need to Know.
Background
Achalasia is a debilitating chronic condition of the esophagus. Contemporary population-based epidemiologic estimates of incidence, prevalence, health care utilization, and costs are needed.
Findings
Two parallel cohort studies conducted using administrative claims data from commercially insured patients and the Medicare population found higher than expected incidence, prevalence, and utilization; burden increased with patient age.
Implications for patient care
The estimates originating from this study suggest that achalasia may not be as rare as previously thought. Gastroenterologists should be keep this condition on their differential diagnosis in the clinic.
Grant Support:
The project described was supported by the National Institutes of Health, through Grant Award Number T32DK007634 (CEG) and P30DK034987. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- POEM
peroral endoscopic myotomy
- APC
annual percent change
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pandolfino JE, Gawron AJ. Achalasia: A Systematic Review. Jama. 2015;313(18):1841–1852. doi: 10.1001/jama.2015.2996 [DOI] [PubMed] [Google Scholar]
- 2.Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet Lond Engl. 2013;383(9911):83–93. doi: 10.1016/s0140-6736(13)60651-0 [DOI] [PubMed] [Google Scholar]
- 3.Gyawali CP. Achalasia: new perspectives on an old disease. Neurogastroenterol Motil. 2016;28(1):4–11. doi: 10.1111/nmo.12750 [DOI] [PubMed] [Google Scholar]
- 4.Pandolfino JE, Kahrilas PJ. Presentation, Diagnosis, and Management of Achalasia. Clin Gastroenterol H. 2013;11(8):887–897. doi: 10.1016/j.cgh.2013.01.032 [DOI] [PubMed] [Google Scholar]
- 5.Nenshi R, Takata J, Stegienko S, et al. The Cost of Achalasia: Quantifying the Effect of Symptomatic Disease on Patient Cost Burden, Treatment Time, and Work Productivity. Surg Innov. 2010;17(4):291–294. doi: 10.1177/1553350610376392 [DOI] [PubMed] [Google Scholar]
- 6.Harvey PR, Thomas T, Chandan JS, et al. Incidence, morbidity and mortality of patients with achalasia in England: findings from a study of nationwide hospital and primary care data. Gut. 2018;68(5):790–795. doi: 10.1136/gutjnl-2018-316089 [DOI] [PubMed] [Google Scholar]
- 7.Leeuwenburgh I, Scholten P, Alderliesten J, et al. Long-Term Esophageal Cancer Risk in Patients With Primary Achalasia: A Prospective Study. Am J Gastroenterol. 2013;108(10):S56. doi: 10.1038/ajg.2010.263 [DOI] [PubMed] [Google Scholar]
- 8.Vaezi MF, Pandolfino JE, Yadlapati RH, Greer KB, Kavitt RT. ACG Clinical Guidelines: Diagnosis and Management of Achalasia. Am J Gastroenterol. 2020;115(9):1393–1411. doi: 10.14309/ajg.0000000000000731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khashab MA, Vela MF, Thosani N, et al. ASGE guideline on the management of achalasia. Gastrointest Endosc. 2019;91(2):213–227.e6. doi: 10.1016/j.gie.2019.04.231 [DOI] [PubMed] [Google Scholar]
- 10.Kumbhari V, Khashab MA. Peroral endoscopic myotomy. World J Gastrointest Endosc. 2015;7(5):496–509. doi: 10.4253/wjge.v7.i5.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vela MF, Richter JE, Khandwala F, et al. The Long-term Efficacy of Pneumatic Dilatation and Heller Myotomy for the Treatment of Achalasia. Clin Gastroenterol H. 2006;4(5):580–587. doi: 10.1016/s1542-3565(05)00986-9 [DOI] [PubMed] [Google Scholar]
- 12.Kahrilas PJ, Pandolfino JE. Treatments for achalasia in 2017. Curr Opin Gastroen. 2017;33(4):270–276. doi: 10.1097/mog.0000000000000365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponds FA, Fockens P, Lei A, et al. Effect of Peroral Endoscopic Myotomy vs Pneumatic Dilation on Symptom Severity and Treatment Outcomes Among Treatment-Naive Patients With Achalasia. Jama. 2019;322(2):134–144. doi: 10.1001/jama.2019.8859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javed AT, Batte K, Khalaf M, Abdul-Hussein M, Elias PS, Castell DO. Durability of pneumatic dilation monotherapy in treatment-naive achalasia patients. Bmc Gastroenterol. 2019;19(1):181. doi: 10.1186/s12876-019-1104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner YB, Hakanson B, Martinek J, et al. Endoscopic or Surgical Myotomy in Patients with Idiopathic Achalasia. New Engl J Medicine. 2019;381(23):2219–2229. doi: 10.1056/nejmoa1905380 [DOI] [PubMed] [Google Scholar]
- 16.Hung Y-C, Westfal ML, Chang DC, Kelleher CM. Heller myotomy is the optimal index procedure for esophageal achalasia in adolescents and young adults. Surg Endosc. 2018;33(10):3355–3360. doi: 10.1007/s00464-018-06625-6 [DOI] [PubMed] [Google Scholar]
- 17.Jacobson BC, Lichtenstein DR. Peroral Endoscopic Myotomy (POEM) vs Pneumatic Dilation: Establishing a New Therapeutic Option for Achalasia. Jama. 2019;322(2):119–120. doi: 10.1001/jama.2019.8858 [DOI] [PubMed] [Google Scholar]
- 18.Samo S, Carlson DA, Gregory DL, Gawel SH, Pandolfino JE, Kahrilas PJ. Incidence and Prevalence of Achalasia in Central Chicago, 2004–2014, Since the Widespread Use of High-Resolution Manometry. Clin Gastroenterol H. 2017;15(3):366–373. doi: 10.1016/j.cgh.2016.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil. 2010;22(9):e256–e261. doi: 10.1111/j.1365-2982.2010.01511.x [DOI] [PubMed] [Google Scholar]
- 20.IBM MarketScan Research Databases for Life Sciences Researchers. Published online 2016. Accessed 21AD. https://www-01.ibm.com/common/ssi/cgi-bin/ssialias?htmlfid=HLW03049USEN&
- 21.Health Research Data for the Real World: The MarketScan Databases. Published online 2014. http://truvenhealth.com/your-healthcare-focus/life-sciences/data-databases-and-online-tools
- 22.Rassen JA, Bartels DB, Schneeweiss S, Patrick AR, Murk W. Measuring prevalence and incidence of chronic conditions in claims and electronic health record databases. Clin Epidemiology. 2018;11:1–15. doi: 10.2147/clep.s181242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehlers AP, Oelschlager BK, Pellegrini CA, et al. Achalasia Treatment, Outcomes, Utilization, and Costs: A Population-Based Study from the United States. J Am Coll Surgeons. 2017;225(3):380–386. doi: 10.1016/j.jamcollsurg.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. Journals Gerontology Ser. 2017;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulaylat A, Schaefer E, Messaris E, Hollenbeak C. Truven Health Analytics MarketScan Databases for Clinical Research in Colon and Rectal Surgery. Clin Colon Rect Surg. 2019;32(01):054–060. doi: 10.1055/s-0038-1673354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghaferi AA, Dimick JB. Practical Guide to Surgical Data Sets: Medicare Claims Data. Jama Surg. 2018;153(7):677. doi: 10.1001/jamasurg.2018.0489 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.