Abstract
The development of a tracheal graft to replace long-segment defects has thwarted clinicians and engineers alike for over 100 years. To better understand the challenges facing this field today, we have consolidated all published reports of engineered tracheal grafts used to repair long-segment circumferential defects in humans, from the first in 1898 to the most recent in 2018, totaling 290 clinical cases. Distinct trends emerge in the types of grafts used over time, including repair using autologous fascia, rigid tubes of various inert materials, and pretreated cadaveric allografts. Our analysis of maximum clinical follow-up, as a proxy for graft performance, revealed that the Leuven protocol has a significantly longer clinical follow-up time than all other methods of airway reconstruction. This method involves transplanting a cadaveric tracheal allograft that is first prevascularized heterotopically in the recipient. We further quantified graft-related causes of mortality, revealing failure modes that have been resolved, and those that remain a hurdle, such as graft mechanics. Finally, we briefly summarize recent preclinical work in tracheal graft development. In conclusion, we synthesized top clinical care priorities and design criteria to inform and inspire collaboration between engineers and clinicians toward the development of a functional tracheal replacement graft.
Impact statement
The field of tracheal engineering has floundered in recent years due to multiple article retractions. However, with recent advances in biofabrication and tissue analysis techniques, the field remains ripe for advancement through collaboration between engineers and clinicians. With a long history of clinical application of tracheal replacements, engineered tracheas are arguably the regenerative technology with the greatest potential for translation. This work describes the many phases of engineered tracheal replacements that have been applied in human patients over the past 100 years with the goal of carrying forward critical lessons into development of the next generation of engineered tracheal graft.
Keywords: tracheal engineering, clinical outcomes, scaffold design, history
Introduction
Tracheal damage has posed a significant challenge to clinicians for decades due to inherent difficulties attendant to any surgical intervention on the airway. The first reported case of tracheal repair in a human was by Koenig in 1896, using a two-step operation to graft a piece of rib and connective tissue across a tracheal fistula.1 Long-segment tracheal defects, typically defined as being over 5 cm long in adults and shorter segments in children, are particularly difficult to address given the poor blood supply of the trachea, constant exposure of the airway lumen to the environment, and complex mechanical demands on the tissue. The first long-segment circumferential repair of the trachea occurred in 1898 and was performed by Bruns, who used a “trachea cannula” of unspecified material to replace 10 tracheal rings in a patient, who went on to live with the repair for 5 years.2
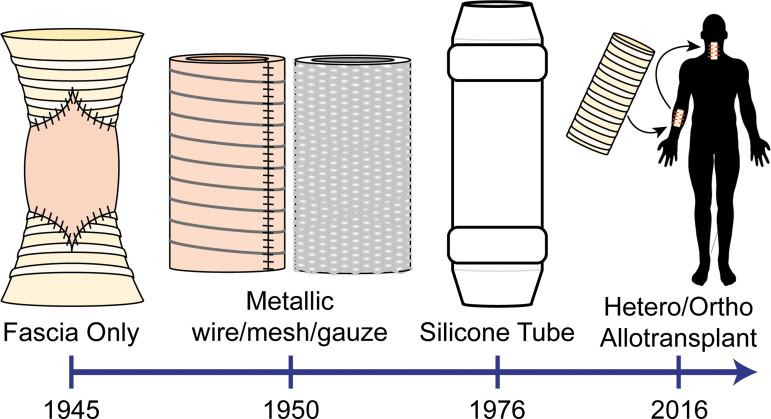
Large tracheal defects may be caused by cancer, trauma, infection, and congenital or acquired stenosis. For more than a century, clinicians have bridged these defects in critically ill patients using a variety of tracheal replacement grafts (Fig. 1). Graft designs have improved over time with advancing material fabrication techniques, surgical procedures, and understanding of tracheal mechanics and physiology, which have generally led to increasing patient survival. Nonetheless, the ideal tracheal replacement, which functions reliably in the long term, remains out of reach.
FIG. 1.
Overview of evolution of long-segment tracheal repair methods over time.
The complex problem of airway reconstruction is ripe for advancement through collaboration between clinicians and engineers. A successful tracheal graft will require both the thoughtful and deliberate fabrication of a functional replacement conduit, as well as skilled surgical techniques and patient postoperative care. With several engineering research groups conducting promising preclinical studies of new types of grafts, physician surgical expertise will be required for clinical translation, so that these advances may reach patients safely and effectively. Although the perception of tracheal engineering has been marred in recent years by reports of ethical lapses by a few investigators, the field continues to show promise and there remains significant clinical need.
The goal of this review is to consolidate all published reports of engineered tracheal grafts used to repair long-segment circumferential defects in humans, from the first in 1898 to the most recent in 2018. From this comprehensive review, we then identify and analyze historical trends in scaffold types, outcomes, and patient mortality. These trends provide clear insights into critical engineering design considerations and clinical approaches that contribute to success in human patients. We hope this review will inform the design and execution of the next generation of engineered tracheal grafts as we approach a viable solution for those suffering from airway defects.
By thorough literature search (PubMed), we identified published cases of tracheal replacement between 1898 and 2018, amounting to 290 patients in total. The scope of clinical cases in this review includes all long-segment circumferential tracheal replacements, excluding patch repairs. Retracted articles were excluded. From each report, we tabulated standardized pertinent information whenever possible, including the year of the published report; principal investigator; country, city, and hospital of transplant; patient age and sex; clinical condition; scaffold type; complications; maximum follow-up; and failure mode. This information has been included in Supplementary Table S1. This information was further synthesized to identify and quantify overarching trends.
Engineered Tracheal Replacements Over Time
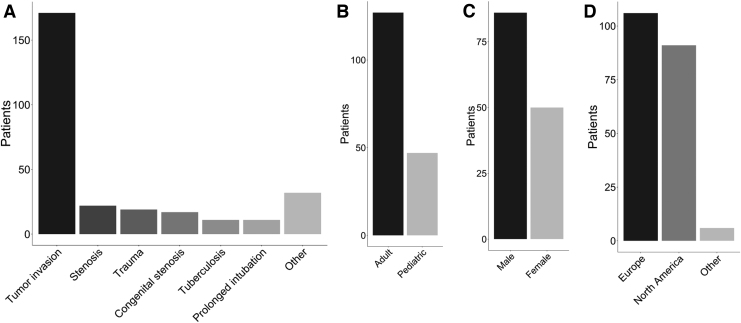
Over 120 years, there were published reports of 290 patients receiving long-segment, circumferential tracheal replacement grafts. While all patients were treated on an urgent need and/or compassionate use basis, the breakdown of reported treatment conditions is as follows: 171 (60.4%) tumor invasion; 22 (7.8%) critical airway stenosis; 19 (6.7%) airway trauma; 17 (6.0%) congenital stenosis; 11 (3.9%) tuberculosis; 11 (3.9%) prolonged intubation; and 32 (11.3%) other conditions (Fig. 2A). One hundred twenty-seven patients (73%) were adults, while 47 patients (27%) were pediatric (younger than 18 years) (Fig. 2B). In cases of pediatric airway reconstruction, consideration must be made for the growth of the patient, which may necessitate further interventions or repeated grafting, in the case of inert implants. To avoid the need for re-intervention, in some cases, adult-sized tracheal grafts have been applied in children.3,4 Eighty-six (63.2%) patients were male and 50 (36.8%) were female (Fig. 2C). One hundred six patients (52.2%) were treated in Europe, while 91 (44.8%) were treated in North America, and 6 (3.0%) were treated elsewhere in the world (Fig. 2D). This patient information was incomplete in some reports. Figure 3 provides a timeline of the types of grafts reported.
FIG. 2.
Patient characteristics. Histograms of (A) patient condition underlying the need for tracheal replacement, (B) patient age, (C) patient sex, and (D) geographic locations of airway procedures.
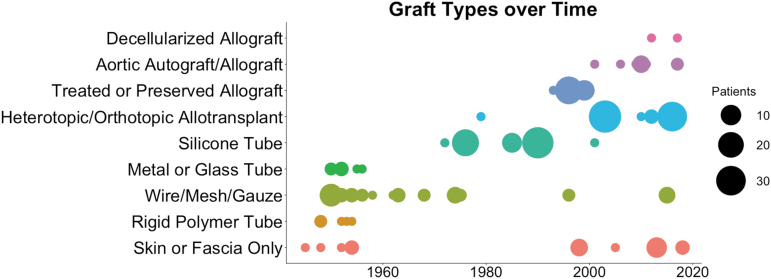
FIG. 3.
Clinical application of graft types over time. Dot plot of the number of patients receiving each type of tracheal replacement graft.
Skin or fascia only
The first grafts developed for long segment circumferential tracheal damage were conduits created from the patient's own tissue (Fig. 3). These graft fabrication techniques varied widely, utilizing autologous dermal graft patches from the thigh, forearm, or chest,1,2,5 and sometimes reinforcing with autologous rib, costal cartilage, or portions of the resected trachea (Fig. 4A).2,6–10 In a recent version,7 a fasciocutaneous flap was harvested from the patient's forearm with the radial artery and vein intact. Six or seven autologous cartilage segments from the caudal ribs were then inserted between the skin and fascia of the graft, without interrupting the vascular bed. The construct was then rolled with the skin oriented luminally, and the ends of the costal cartilage sutured together to form a tubular conduit. When the graft was anastomosed to the ends of the remaining native trachea, the radial vessels were also microscopically anastomosed to small neck vessels, thereby restoring vascularization to the graft. While these grafts avoid the need for foreign materials or immunosuppression, and often revascularize well, they typically required interventions to remove secretions, and dilations and stentings to remediate stricture and collapse. This type of autologous scaffold has been used the longest of all graft types, from the first report in 1927 to the most recent in 2018 (Fig. 3). Nonetheless, this method has never gained wide acceptance over that time, likely because of the complexity of graft fabrication, the morbidity of multiple wound sites, and inconsistent clinical outcomes.
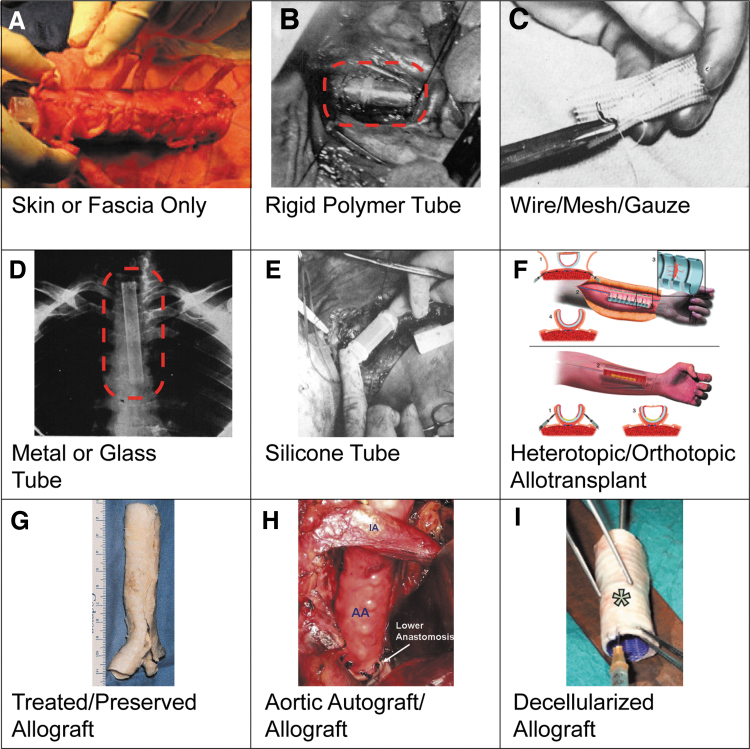
FIG. 4.
Examples of scaffold types used in patients. Gross images of each type, adapted from cited publications: (A) skin or fascia only (Fabre et al.7), (B) rigid polymer tube (Clagett et al.12), (C) wire/mesh/gauze (Ellis et al.27), (D) metal (or glass) tube (Cotton and Penido2), (E) silicone tube (Neville et al.38), (F) heterotopic/orthotopic allotransplant (Delaere et al.52), (G) treated or preserved allograft (Jacobs et al.3), (H) aortic autograft/allograft (Wurtz et al.60), and (I) decellularized allograft (Elliott et al.65). Red dotted boxes added to aid visualization of graft. Images reproduced from cited articles with permissions from Elsevier, Wiley, and associated journals.
Rigid polymer tube
To avoid the airway narrowing and collapse of soft fascial-derived grafts, some clinicians began implanting rigid polymeric tubes as replacement airways (Fig. 3). These grafts were made of either polyethylene (Fig. 4B)11–13 or poly(methyl methacrylate),14,15 which is commonly used to repair bony defects. The rigid tubes were secured at the anastomoses with either purse string sutures11 or interrupted sutures through the prosthesis, where possible.13 Being quite stiff, these solid polymeric grafts consistently remained patent. However, these tubes often shifted out of place to partially or fully block bronchi or entire lung lobes, due to the difficulty of suturing securely, resulting in pneumonia or death.11 There is no reported clinical application of these grafts after 1954 (Fig. 3).
Wire/mesh/gauze
Another common early engineered replacement graft utilized metallic or polymeric wire, mesh, or gauze (Fig. 3). These grafts were intended to strike a middle ground between overly soft fascial/skin grafts and overly rigid polymeric grafts, by resisting airway lumen collapse, while enabling some flexibility essential to normal physiologic head and neck movements. These scaffolds were constructed from tantalum gauze,1,15–17 stainless steel wire/mesh,12,16,18–25 rods15 or springs,23,26 Marlex mesh (Fig. 4C),24,27–32 or titanium-nickel alloy stent.33 These materials were selected to be minimally corrosive and relatively inert on implantation. In most cases, these constructs were wrapped in fascia to render the conduit airtight.
While some of these grafts provided functional airways to patients for up to several years, there was also significant morbidity associated with these methods. The main complications with this class of graft were formation of granulation tissue at the anastomoses12,20,29 or through the graft,17,24 or airway edema around the scaffold materials.22,33 The wire/mesh/gauze constructs also were not perfect mechanical solutions, as many suffered airway narrowing and collapse.12,18–20,22,24 The primary graft-related cause of mortality associated with these constructs was migration of sharp edges of the graft into neighboring vascular structures, such as the innominate artery, leading to hemorrhage.15,16,26,30,32 To mitigate the risk of hemorrhage for this and other types of grafts, clinicians began to employ fascia grafts or other materials to insulate major vascular structures from the tracheal graft.27 The second most common cause of mortality was blockage of the airway by secretions.23,24 Although never consistently safe and effective, this type of graft has the second longest reported run following autologous fascia/skin grafts, from 1949 to 2015 (Fig. 3).
Metal or glass tube
Similar to the rigid polymeric tubes, metal or glass tubes were also applied in early years as reliably patent tracheal replacements, but are only reported from 1950 to 1956 (Fig. 3). These tubes were made of stainless steel (Fig. 4D),2,20,23 vitallium,20 or Pyrex glass.34 The stainless steel tubes were secured with wire sutures through holes in the ends of the tube, and admittedly were not perfectly airtight.2 Pyrex tubes were flanged at the ends and absorbable sutures were used around the flange, and then a pedicle pleural graft was sutured over the anastomosis to seal the construct.34 These grafts were hampered by migration into the innominate artery or obstruction of bronchi,2,23 similar to rigid polymeric tubes. The use of opaque, inert tubes also generally precluded bronchoscopic evaluation of regenerating host tissue, and did not support the ingrowth of native epithelium.
Surgical advances
There is a notable gap in reports of clinical tracheal replacement grafts starting in the 1960s (Fig. 3). During that time, advances in surgical techniques extended the length of trachea that could be repaired by primary anastomosis from 2 cm to more than 50% of the length of the trachea in adults, by using new tension-relieving maneuvers.35 These tracheal release procedures are still used today, and include dissection of the pretracheal plane, cervical flexion, laryngeal release, and hilar release techniques. In an interesting case, these methodologies were applied to a patient who originally received a Marlex mesh tracheal replacement in 1963,29 which functioned well for 5 years until the patient was re-operated for a local tumor recurrence, at which time the graft was removed and primary ends of the trachea re-anastomosed following the new tissue releases. This patient survived almost 30 more years until she died of another local recurrence.36
Silicone tube
The next generation of tracheal replacement graft sought to resolve issues associated with overly rigid constructs, without compromising on patency and potentially allowing for more mechanical flexibility. Silicone rubber was employed for its durability, low tissue reactivity, and ability to be molded into various sizes and configurations. Tubular silicone airway grafts were implanted in either straight or bifurcated conformations to replace the trachea and mainstem bronchi (Fig. 4E).37–41 The outside ends of the tubes were often functionalized with Dacron to enhance suturability and integration with host tissue.37 Nonetheless, the main issue with these grafts was shifting and loosening of the prosthesis,38–40 which sometimes led to hemorrhage.38,39 Granuloma formation at the anastomoses was also an issue.38–40 This phase of tracheal graft was introduced following extensive preclinical testing by Dr. William Neville, who reported the first clinical application of a silicone tube as an airway replacement in 1972.37 He went on to implant 59 patients with silicone tracheal prostheses until 1990, accounting for 85% of all clinical applications of this technology. At their peak usage, silicone tubes were the graft with the most reported clinical applications, but showed little improvement in survival over wire/mesh/gauze implants (Fig. 5). This method has not been reported since 2001, likely due to the lack of constructive integration with host tissue (Fig. 3).
FIG. 5.
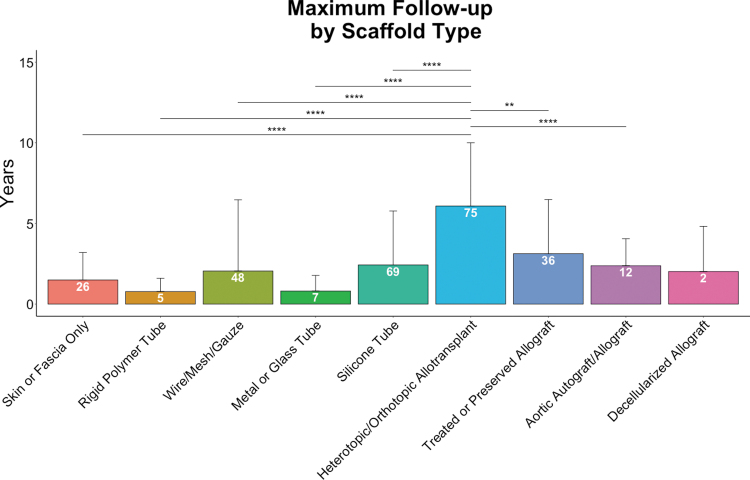
Maximum reported follow-up time by tracheal graft type. Pairwise statistical comparisons were made by Welch's t-tests (**p < 0.01, ****p < 0.0001). Number of patients per condition (n) indicated on data bars in white. Error bars represent SEM. SEM, standard error of the mean.
Treated or preserved allografts
Advances in immunosuppressive therapies in the 1980s and 1990s led to critical improvements in survival for solid organ and composite tissue transplants, including the first successful lung transplantations.42–44 The first successful laryngotracheal transplant occurred in 1998.45 This surgical advance enhanced the prospects of tracheal allotransplantation in theory, but transplanting tracheas has remained more complicated in practice. Airway allografts often became ischemic and/or necrotic by 2 months post-transplantation, and sometimes required stenting to remain patent. For these reasons, direct allografting from donor to recipient has been largely abandoned clinically, with only anecdotal reports in recent years.46
Initially, the cause of tracheal allograft necrosis was attributed to adverse immune response. In efforts to enhance graft viability at the time of operation, one tracheal allograft was treated with Eurocollins solution.47 In this case, the graft began to be rejected and necrose by day 10, thereby defeating the purpose of treatment. By 4 months, the graft progressed to stenosis requiring repeated stenting to prevent collapse. Another approach was to preserve the tracheal allografts in formalin, to both render the tissue immunologically inert and to impart some stiffening of the tissue to avoid collapse (Fig. 4G).3,4,48 Notably, this type of preserved allograft was employed in about half of all pediatric cases of airway replacement, 24 out of 47, at Great Ormond Street, Hospital for Children (GOSH) in London, United Kingdom.3,48 In these cases, an intraluminal silicone stent supported the graft for 10–12 weeks until the graft reepithelialized, at which point the stent was removed. Most of these grafts performed well in the short and medium term. A major benefit to using fixed cadaveric allografts is their shelf life, which allows them to be used in emergency cases or without a long lead time. Nonetheless, a number of fixed tracheal allografts still suffered stenosis and required stenting to maintain patency in the long term.3,4,47,48 Consequently, preserved and/or fixed tracheal allografts have likely been abandoned as a viable replacement airway conduit, having only been reported from 1993 to 1999 (Fig. 3).
Heterotopic/orthotopic allotransplant
Some investigators believed that ischemia and necrosis observed in direct allotransplants were primarily due to insufficient revascularization in vivo, rather than immunological rejection. The postulate was that prevascularization of the airway implant, instead of chemical fixation, could ameliorate necrotic failure. By this method, allogeneic tracheas were initially implanted into a highly vascularized region of the recipient, before orthotopic transplantation to the relatively perfusion-poor region where the trachea resides. A brief early report on this method in 1979 described a successful tracheal allotransplant following heterotopic revascularization of the graft in the patient's sternocleidomastoid muscle, without immunosuppression.49 This heterotopic/orthotopic allotransplantation method continued to be honed by Dr. Pierre Delaere over years of preclinical studies to the point of a reliable protocol suitable for humans. Delaere has since implanted 72 patients with the so-called Leuven protocol, which is named after the hospital where he developed the method.
In the Leuven protocol, a cadaveric allograft is revascularized heterotopically in the recipient (usually in the forearm) for 2–4 months before orthotopic transplantation (Fig. 4F).50–57 Daily triple immunosuppression is usually initiated upon heterotopic implantation of the graft and continues for 15–18 months following orthotopic transplantation, using tacrolimus (Prograf), mycophenolate mofetil (Cellcept), and methylprednisolone.54 It has also been found that removal of the trachealis muscle and creation of intercartilaginous incisions both aid neovascularization within the graft, although it has yet to be determined how these alterations affect the overall mechanical properties of the graft. The Leuven protocol has also been applied in two additional cases by other clinicians at different hospitals in Europe.56,57 In addition to being the method of long-segment circumferential tracheal replacement with the greatest number of reported clinical cases (n = 75), it also has the longest reported follow-up time, making it the current gold standard in the field (Fig. 5). The main complications associated with this method are stricture,52 tracheocutaneous fistula occurring in the forearm,55 and infection at both the forearm wound site and following orthotopic tranplantation.55 There are also reported cases where the method failed completely when the graft necrosed in the forearm.52 The Leuven protocol is ongoing in the clinic, with the most recent case report in 2016 (Fig. 3).
Aortic autograft/allograft
Another type of natural graft applied clinically since 2001 utilizes aorta as a replacement conduit (Fig. 3). In this procedure, a fresh or cryopreserved aortic graft is stented and implanted as an airway replacement (Fig. 4H).58–63 In cases of autotransplantation, a portion of the patient's abdominal aorta is first resected and replaced with a vascular graft.58,59,62 This grafting method was developed for emergency situations where no other airway conduit was available, and was not originally intended as a long-term solution.58 Since the aorta is a vascular conduit intended to sustain positive intraluminal blood pressure, it is not a mechanically adequate substitute for the trachea, which must withstand the negative intraluminal pressures of breathing, coughing, and sneezing. For this reason, aortic grafting into the airway requires stenting, which has led to complications and morbidity associated with stent granulation and migration.60–62 In addition, anastomotic dehiscence has been reported.59,60 Evaluation of cryopreserved, stented aortic allografts for airway repair in lung cancer surgery is the basis of an ongoing clinical trial in France beginning in 2017, under Dr. Emmanuel Martinod (TRACHEOBRONCART, NCT01331863).61 In the two reported cases so far, both patients were alive at 55 and 67 months post-transplant. While the grafts showed some preliminary signs of integrating with the host, both also faced complications related to the stents, including stent bacterial infection and granuloma.
Decellularized allograft
Another method of allotransplantation leverages modern decellularization techniques to remove all cellular components from cadaveric tracheas, using a combination of detergents and enzymes, leaving behind only extracellular matrix proteins. The goal of this decellularization process is to render the tissue immunologically inert.64 This method has been reported in two clinical cases since 2012, both in children (Fig. 4I).65–67 One patient has undergone 25 follow-up procedures in 4 years to clear secretions and to stent the collapsing allograft,65,67 and the other patient died 15 days postoperatively of graft collapse.66 In the surviving patient, evaluation of the graft by computed tomography scanning over time reveals graft luminal narrowing coupled with dilation of native tissue at the anastomoses, consistent with mechanical mismatch of the graft and remaining trachea, possibly related to graft collapse or adverse host response.67 In follow-up in vitro analyses, it has been demonstrated that the decellularization protocol applied in these clinical cases significantly reduces glycosaminoglycan and collagen content of the tissue, and significantly impairs circumferential cartilage ring rigidity and resistance to collapse.68,69 Decisions to use decellularized allografts in these two cases were largely based on work that has come under heavy criticism for misrepresentation of clinical outcomes, resulting in ongoing legal proceedings and multiple article retractions.
Macchiarini controversy
During the last decade, the field of clinical tracheal replacements became mired in global controversy surrounding the work of Dr. Paolo Macchiarini, who has been involved in the clinical implantation of multiple decellularized and polymeric airway replacements since 2008. Despite insufficient preclinical validation of the safety and efficacy of these techniques, Macchiarini's human implantations were broadly espoused, and his reported results were used as the basis for clinical application by other groups. Macchiarini's work was lauded as the first application of “stem cell technology” in humans, despite offering no evidence of effective application of true stem or progenitor cell sources. The outsized praise for the work of Dr. Macchiarini has since collapsed as he, his colleagues, and a number of members of the Karolinska Institute have been found guilty of a variety of ethical lapses.70 The scale of publicity surrounding this technology has meant a boom and bust in public perception not only of the field of tracheal replacement but also more broadly of all of regenerative medicine. Recent retrospective studies have also uncovered the true mortality and suffering of his patients.71 Due to ongoing ethical investigations and article retractions, Dr. Macchiarini's published work is not included in this review.
Scaffold Outcomes
Defining the current gold standard
For this review, we defined “maximum follow-up” as the longest published follow-up period for each patient post-transplant, whether that was a follow-up report on a living patient or the survival time of a deceased patient. We considered “maximum follow-up” time as a proxy for comparing the performance of different tracheal grafts over time, since longer follow-up times generally indicate better patient survival. This metric does not take into consideration graft complications or patient morbidity, nor does it account for parameters regarding patient selection. It is important to note that essentially all of the tracheal replacement cases included in this review were performed under compassionate use protocols, and not under true prospective clinical trials. Because each case involved a patient in dire need of an airway replacement, and who often had concomitant medical conditions, many of these patients died of causes unrelated to their tracheal grafts. Despite the limitations in the reporting of this metric, statistically significant trends still emerge when comparing different types of tracheal replacements.
Heterotopic/orthotopic allotransplantation using the Leuven protocol has not only been applied to the greatest number of patients (n = 75) but also has the greatest average maximum follow-up, at 6.08 ± 3.92 years (Fig. 5). This is significantly greater (by pairwise Welch's t-test) than the maximum follow-up for nearly all other scaffold types. For this reason, we believe the Leuven protocol should be considered the current gold standard in clinical tracheal transplantation. However, this method is still limited to patients whose physical condition can tolerate the morbidity associated with multiple wound sites and long total procedure times, and it also requires some degree of systemic immunosuppression.
Tracheal graft-related mortality
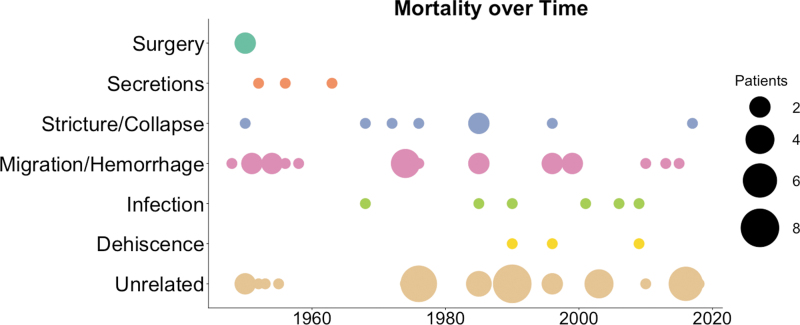
Observing trends in graft-related cause of death generates a portrait of the minimum critical design requirements for a successful tracheal graft (Figs. 6 and 7). Again, observing mortality alone does not take into consideration graft complications or associated patient morbidity, and is limited by the number of cases that report cause of death. Patient deaths reported as unrelated to the tracheal graft procedure or function are also included for completeness (Fig. 6). Despite inherent limitations of this analysis, useful patterns in failure modes emerge.
FIG. 6.
Causes of mortality in patients receiving tracheal replacement grafts.
FIG. 7.
Critical care priorities and design criteria for a successful engineered trachea, synthesized from clinical experience summarized in this review, and compared to the Grillo Design Criteria.90
Several of the early causes of death related to engineered tracheal grafts have been remedied to the point that they are no longer fatal (Fig. 6). Early on, several patients died on the operating table before receiving their graft, due to surgical or anesthetic complications.22 This was the first cause of morbidity to be resolved as the procedures for tracheal transplant rapidly improved. Later, a few early patients also died of suffocation by airway secretions that accumulated either proximal or distal to the repair.22–24,72 Inspissation and blockage from secretions could occur as early as 2–3 days after implantation, and may have been worsened by local infection or adverse host response to the implant materials. To resolve this issue, it quickly became standard follow-up procedure to monitor patients for excess mucous and regularly remove buildup by blind suctioning or bronchoscopic techniques.
Another early cause of death was narrowing and collapse of the tracheal graft lumen, which can lead to ventilatory insufficiency and suffocation.2,3,22,29,37–39,66 Airway graft stricture generally occurs by two mechanisms, either fibrotic narrowing due to host response or collapse due to insufficient mechanical integrity. Unfortunately, this distinction is not usually specified in published reports. Airway luminal narrowing as a function of fibrotic host response and inflammation can occur over weeks to months, and can be treated with bronchoscopic dilations and graft stenting to maintain patency, despite encroaching fibrotic tissue. A better long-term solution would be to address the causes of adverse host response before implantation, and to encourage appropriate integration with native host tissue to avoid the need for dilations and stentings.
Graft collapse due to insufficient mechanics is mostly unique to the repair of long-segment circumferential defects. In contrast to patch grafts on the airway, circumferential engineered grafts must have adequate stand-alone mechanical integrity, rather than relying on existing cartilaginous rings, trachealis muscle, and connective tissue structures for support. For this reason, it is preferable to avoid complete circumferential resection of the trachea wherever possible, to better preserve native-like mechanical integrity and avoid graft collapse. Mechanical failure of grafts on implantation can be avoided by only implanting grafts having adequate mechanical properties to avoid collapse, as assessed by mechanical testing and validation under physiologic conditions before implantation. While many airway replacement scaffolds underwent some degree of preclinical testing in vivo before being applied in human patients, very few underwent specific quantitative mechanical testing, and there is not yet a robust and standardized method for doing so.
It has become standard procedure to apply luminal stents to tracheal grafts to prevent stricture or collapse, yet this practice introduces significant risks and morbidity. The introduction of metallic or polymeric stents into the airways increases the risk of graft and/or stent migration, and often causes obstructive stenosis and granulation tissue requiring repeated interventions.4,5,60–62,65 In some cases, degradable stents have been utilized with no mechanism by which to encourage the development of sufficient mechanical integrity of tracheal grafts, leading to repeated stentings as the degradable stents lose their functionality.65 An ideal tracheal replacement graft would avoid the need for stents altogether, by being engineered with sufficient mechanical integrity to maintain patency over time, as well as integrate with the host after implantation so as to avoid narrowing due to adverse host response.
Another significant challenge plaguing the field is tracheal graft migration into neighboring vascular structures, most commonly into the innominate artery, leading to rapid death by exsanguination. This complication has been exacerbated by grafts with sharp edges that incorporate metal wire, mesh, springs, or stents, or grafts that are themselves supraphysiologically rigid. This type of fatal graft migration has occurred anywhere from 10 days23,30 to 6 weeks31 after implantation. Occasionally, rigid grafts migrate into the tracheal lumen and block the airways themselves, leading to airway obstruction and patient death.11,15 While any tracheal graft must be rigid enough to remain patent, it must also be flexible enough to readily and safely accommodate a broad range of head, neck, and chest movements.
With the resolution of some early graft-related causes of death by the mid-1960s, a second wave of fatal failure modes has arisen over the last 50 years (Fig. 6). These secondary causes of mortality appear to derive from declining graft function over time as patients live longer and resume normal lives. The first emerging cause of mortality has been infection, which includes pneumonia40,62 and mediastinitis.41,59 While local, intra-airway infections are not often fatal, they usually appear within days following the transplant and progress rapidly.41,59 Pneumonia can appear anywhere from days11,32 to years40,55,60 after airway transplant. It is critical to closely monitor patients for signs of infection after tracheal replacement, particularly in cases where patients are immunocompromised.
The most recent failure mode to emerge has been graft anastomotic dehiscence, which has occurred anywhere from days32,59 to years40 after implant. Dehiscence often leads to airway obstruction, mediastinitis, and death. Suturability of any airway implant and long-term integrity of the anastomosis are an essential consideration in graft design. Since dehiscence can be caused by necrosis of the tissue at the anastomoses, or by mechanical failure of implanted or native tissues, many engineered constructs have added cuffs or other adaptations to facilitate strong, air-tight anastomoses. It will continue to be important to test graft materials for suture retention going forward, including particular observation of suture strength over time after grafts have been implanted in vivo.
Preclinical Promise
Outside the clinic, the next generation of engineered tracheal replacement graft is being developed in research laboratories and tested in animals. A number of groups are addressing challenges seen in the clinic through focused and well-controlled preclinical work. Recent advancements in tracheal grafts are broadly focused on preventing adverse host response,73–75 capturing complex native mechanical behavior,76–81 and improving functional integration upon implantation.76,82–85 The Cho group of South Korea has developed a polymeric tracheal replacement graft designed with a “bellows” configuration, comprising polycaprolactone, to attempt to capture native-like structure and mechanical behavior.77 The host response to the bellows-type implant was not significantly more inflammatory than a syngeneic allograft.73
Our group has developed a tracheal graft that combines engineered natural scaffolding with nondegradable stents, and are acellular on implantation.76 The supporting stents are encased in collagenous tissue generated by smooth muscle cells in culture before the constructs are decellularized, leveraging the same technology developed over more than two decades for engineered vascular grafts.86 Currently, these engineered arteries are undergoing Phase III clinical trials for vascular replacement (HUMANITY V006, NCT02644941; HAV-ACCESS V007, NCT03183245).87 Our engineered tracheal constructs have functioned well in rodents and primates for up to 2 months, with no instances of collapse or migration. However, mid-graft stenosis has been observed in some of the grafts, and current research is focused on mitigating this host response.
The Nagayasu group in Japan has tried to avoid complications associated with foreign material scaffolds by generating a scaffold-free construct of 3D-printed multicellular spheroids, which has shown promising regenerative integration on implantation in rats.82 Spheroids consist of chondrocytes, endothelial cells, and mesenchymal stem cells and showed evidence of maturing into organized cartilage after implantation, as well as improved mechanics over time in vivo. Furthermore, the Omori group has conducted a number of studies to promote integration and performance of engineered tracheas, including evaluating graft material composition,85 suturability,84 vascularization,81 and epithelialization.83 They have also reported a few clinical applications of their Marlex mesh/collagen sponge graft material as a patch replacement for small tracheal defects, with promising outcomes.88,89 Overall, there remains a major opportunity for clinicians and engineers to learn from each other and collaborate to develop a tracheal replacement graft that works well for patients in the long term, and which resists the multiple failure modes observed in recent decades.
Conclusions
Based upon this comprehensive review of tracheal replacement grafts and their shortcomings in humans, we have derived key functional attributes that must be incorporated into any tracheal replacement that is to function long term. In terms of clinical care priorities, it was learned early on that tracheal grafts must be implantable with repeatable, reliable surgical techniques. Subsequently, patients must be monitored to avoid potentially life-threatening secretion build-up or graft-related infection postoperatively. The tracheal graft itself must be made of a safe, nonimmunogenic material for implantation to avoid adverse host response that could lead to airway failure. Bulk mechanical properties remain the most significant ongoing challenge to the field of tracheal engineering, with Stricture/Collapse and Migration/Hemorrhage accounting for 68% of graft-related mortality in all clinical cases. Mechanically, the graft must not be so stiff or sharp as to migrate and perforate nearby vascular structures, and not so soft as to collapse and require stenting to remain patent, which can lead to a host of stent-related complications. Finally, the most recent finding is that as patients live longer with their replacements, the graft material must be able to maintain adequate suture strength and develop suitable host incorporation over time, to prevent graft dehiscence at the anastomoses (Fig. 7).
Our design criteria align remarkably well with the tracheal replacement design criteria set forth by Dr. Hermes Grillo in 2002, who is broadly considered to be the father of tracheal surgery (Fig. 7).90 The only requirement Grillo adds that was not a major failure mode based on our analysis is the need for a tracheal replacement graft to support reepithelialization. This would restore physiologic function of the airway, including cleaning and humidifying inhaled air, as well as clearing secretions by cilia movement. While lack of epithelium has not been linked directly to graft failure clinically, it is likely critical for the function of an optimal tracheal graft.
Overall, this comprehensive retrospective analysis of all reported clinical cases of long-segment circumferential tracheal replacements has yielded valuable insights on both the current state of the field, as well as a proposed path toward the next generation of engineered tracheas. Meeting these critical criteria for a successful graft will require concerted effort between clinicians and engineers as we progress toward a safe and effective solution for patients.
Supplementary Material
Authors' Contributions
A.M.G. and L.E.N. conceived, planned, interpreted, and reported data. A.M.G. acquired and analyzed data.
Disclosure Statement
L.E.N. is a founder of and shareholder in Humacyte, which is a regenerative medicine company. Humacyte produces engineered blood vessels from allogeneic smooth muscle cells for vascular surgery. L.E.N.'s spouse has equity in Humacyte, and L.E.N. serves on Humacyte's board of directors. L.E.N. is listed as an inventor on patents that are licensed to Humacyte and that produce royalties for L.E.N. L.E.N. has received an unrestricted research gift to support research in her laboratory at Yale University. Humacyte did not fund these studies and Humacyte did not influence the conduct, description, or interpretation of the findings in this article.
Funding Information
This work was supported by grants from the National Institutes of Health to Laura E. Niklason (1R01 HL138540 and 1R21 EB024889).
Supplementary Material
References
- 1.Rob, C.G., and Bateman, G.H.. Reconstruction of the trachea and cervical oesophagus—preliminary report. Br J Surg 37,202, 1949 [DOI] [PubMed] [Google Scholar]
- 2.Cotton, B.H., and Penido, J.R.F.. Resection of the trachea for carcinoma—report of 2 cases. J Thorac Surg 24,231, 1952 [PubMed] [Google Scholar]
- 3.Jacobs, J.P., Elliott, M.J., Haw, M.P., Bailey, C.M., and Herberhold, C.. Pediatric tracheal homograft reconstruction: a novel approach to complex tracheal stenoses in children. J Thorac Cardiovasc Surg 112,1549, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Jacobs, J.P., Quintessenza, J.A., Andrews, T., et al. Tracheal allograft reconstruction: the total North American and worldwide pediatric experiences. Ann Thorac Surg 68,1043, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Spaggiari, L., Calabrese, L.S., D'Aiuto, M., et al. Successful subtotal tracheal replacement (using a skin/omental graft) for dehiscence after a resection for thyroid cancer. J Thorac Cardiovasc Surg 129,1455, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Backer, C.L., Mavroudis, C., Dunham, M.E., and Holinger, L.D.. Repair of congenital tracheal stenosis with a free tracheal autograft. J Thorac Cardiovasc Surg 115,869, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Fabre, D., Kolb, F., Fadel, E., et al. Successful tracheal replacement in humans using autologous tissues: an 8-year experience. Ann Thorac Surg 96,1146, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Kolb, F., Simon, F., Gaudin, R., et al. 4-Year follow-up in a child with a total autologous tracheal replacement. N Engl J Med 378,1355, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Daniel, R.A.The regeneration of defects of the trachea and bronchi—an experimental study. J Thorac Surg 17,335, 1948 [PubMed] [Google Scholar]
- 10.Thomet, C., Modarressi, A., Ruegg, E.M., Dulguerov, P., and Pittet-Cuenod, B.. Long-segment tracheal reconstruction with free radial forearm flap reinforced by rib cartilage. Ann Plast Surg 80,525, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Clagett, O.T., Grindlay, J.H., and Moersch, H.J.. Resection of the trachea—an experimental study and a report of a case. Arch Surg-Chicago 57,253, 1948 [DOI] [PubMed] [Google Scholar]
- 12.Clagett, O.T., Moersch, H.J., and Grindlay, J.H.. Intrathoracic tracheal tumors—development of surgical technics for their removal. Ann Surg 136,520, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig, R.L., Holmes, G.W., and Shabart, E.J.. Tracheal resection and replacement with a prosthesis. J Thorac Surg 25,384, 1953 [PubMed] [Google Scholar]
- 14.Longmire, W.P.The repair of large defects of the trachea. Ann Otol Rhinol Laryngol 57,875, 1948 [DOI] [PubMed] [Google Scholar]
- 15.Edgerton, M.T., and Zovickian, A.. Reconstruction of the trachea and infraglottic larynx. Plast Reconstr Surg 13,167, 1954 [DOI] [PubMed] [Google Scholar]
- 16.Gebauer, P.W.Reconstructive surgery of the trachea and bronchi—late results with dermal grafts. J Thorac Surg 22,568, 1951 [PubMed] [Google Scholar]
- 17.Cahan, W.G.Carcinoma of intrathoracic trachea—excision and repair by tantalum gauze-fascia lata graft—report of a case. J Thorac Surg 23,513, 1952 [PubMed] [Google Scholar]
- 18.Belsey, R.Resection and reconstruction of the intrathoracic trachea. Br J Surg 38,200, 1950 [DOI] [PubMed] [Google Scholar]
- 19.Gebauer, P.W.Plastic reconstruction of tuberculous bronchostenosis with dermal grafts. J Thorac Surg 19,604, 1950 [Google Scholar]
- 20.Gebauer, P.W.Further experiences with dermal grafts for healed tuberculous stenosis of the bronchi and trachea. J Thorac Surg 20,628, 1950 [PubMed] [Google Scholar]
- 21.Gebauer, P.W.The use of dermal grafts for tuberculous stenosis of the trachea and bronchi. Hawaii Medical J 8,413, 1949 [PubMed] [Google Scholar]
- 22.Gebauer, P.W.Experiences with surgical reconstruction of the trachea. Am Rev Tuberc 62,176, 1950 [DOI] [PubMed] [Google Scholar]
- 23.Keshishian, J.M., Blades, B., and Beattie, E.J.. Tracheal reconstruction. J Thorac Surg 32,707, 1956 [PubMed] [Google Scholar]
- 24.Kramish, D., and Morfit, H.M.. The use of a teflon prosthesis to bridge complete sleeve defects in the human trachea. Am J Surg 106,704, 1963 [DOI] [PubMed] [Google Scholar]
- 25.Grillo, H.C.Tracheal Replacement. Surgery of the Trachea and Bronchi. Hamilton, ON, Canada: BC Decker, Inc., 2004 [Google Scholar]
- 26.Taber, R.E., and Tomatis, L.. Experimental and clinical utilization of a prosthesis for replacement of the trachea. Arch Surg 77,576, 1958 [DOI] [PubMed] [Google Scholar]
- 27.Ellis, P.R., Harrington, O.B., Debakey, M.E., and Beall, A.C.. Use of heavy Marlex mesh for tracheal reconstruction following resection for malignancy. J Thorac Cardiovasc Surg 44,520, 1962 [Google Scholar]
- 28.Beall, A.C., Harrington, O.B., Greenberg, S.D., Morris, G.C., and Usher, F.C.. Tracheal reconstruction with heavy Marlex mesh. Arch Surg 86,970, 1963 [DOI] [PubMed] [Google Scholar]
- 29.Pearson, F.G., Henderson, R.D., Gross, A.E., Ginsberg, R.J., and Stone, R.M.. The reconstruction of circumferential tracheal defects with a porous prosthesis. An experimental and clinical study using heavy Marlex mesh. J Thorac Cardiovasc Surg 55,605, 1968 [PubMed] [Google Scholar]
- 30.Pearson, F.G., Thompson, D.W., Weissberg, D., Simpson, W.J.K., and Kergin, F.G.. Adenoid cystic carcinoma of trachea—experience with 16 patients managed by tracheal resection. Ann Thorac Surg 18,16, 1974 [DOI] [PubMed] [Google Scholar]
- 31.Moghissi, K.Tracheal reconstruction with a prosthesis of Marlex mesh and pericardium. J Thorac Cardiovasc Surg 69,499, 1975 [PubMed] [Google Scholar]
- 32.Maziak, D.E., Todd, T.R.J., Keshavjee, S.H., Winton, T.L., VanNostrand, P., and Pearson, F.G.. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg 112,1522, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Zhang, S.N., and Liu, Z.G.. Airway reconstruction with autologous pulmonary tissue flap and an elastic metallic stent. World J Surg 39,1981, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Abbott, O.A., Vanfleit, W.E., and Roberto, A.E.. Experiences with extending the indications for the use of tracheal and bronchial grafts. J Thorac Surg 29,217, 1955 [PubMed] [Google Scholar]
- 35.Heitmiller, R.F.Tracheal release maneuvers. Chest Surg Clin N Am 13,201, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Deslauriers, J.Birth of airway surgery and evolution over the past fifty years. Thorac Surg Clin 28,109, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Neville, W.E., Andersen, J., Hamouda, F., and Dwan, F.M.. Replacement of intrathoracic trachea and both stem bronchi with a molded silastic prosthesis. J Thorac Cardiovasc Surg 63,569, 1972 [PubMed] [Google Scholar]
- 38.Neville, W.E., Bolanowski, P.J., and Soltanzadeh, H.. Prosthetic reconstruction of the trachea and carina. J Thorac Cardiovasc Surg 72,525, 1976 [PubMed] [Google Scholar]
- 39.Toomes, H., Mickisch, G., and Vogtmoykopf, I.. Experiences with prosthetic reconstruction of the trachea and bifurcation. Thorax 40,32, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neville, W.E., Bolanowski, P.J.P., and Kotia, G.G.. Clinical-experience with the silicone tracheal prosthesis. J Thorac Cardiovasc Surg 99,604, 1990 [PubMed] [Google Scholar]
- 41.Schneider, P., Schirren, J., Muley, T., and Vogt-Moykopf, I.. Primary tracheal tumors: experience with 14 resected patients. Eur J Cardiothorac Surg 20,12, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Reitz, B.A., Wallwork, J.L., Hunt, S.A., et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med 306,557, 1982 [DOI] [PubMed] [Google Scholar]
- 43.Cooper, J.D., Pearson, F.G., Patterson, G.A., et al. Technique of successful lung transplantation in humans. J Thorac Cardiovasc Surg 93,173, 1987 [PubMed] [Google Scholar]
- 44.Cooper, J.D., Patterson, G.A., Grossman, R., and Maurer, J.. Double-lung transplant for advanced chronic obstructive lung disease. Am Rev Respir Dis 139,303, 1989 [DOI] [PubMed] [Google Scholar]
- 45.Strome, M., Stein, J., Esclamado, R., et al. Laryngeal transplantation and 40-month follow-up. N Engl J Med 344,1676, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Delaere, P., Van Raemdonck, D., and Vranckx, J.. Tracheal transplantation. Intensive Care Med 45,391, 2019 [DOI] [PubMed] [Google Scholar]
- 47.Levashov, Y.N., Yablonsky, P.K., Cherny, S.M., Orlov, S.V., Shafirovsky, B.B., and Kuznetzov, I.M.. One-stage allotransplantation of thoracic segment of the trachea in a patient with idiopathic fibrosing mediastinitis and marked tracheal stenosis. Eur J Cardiothorac Surg 7,383, 1993 [DOI] [PubMed] [Google Scholar]
- 48.Jacobs, J.P., Haw, M.P., Motbey, J.A., Bailey, C.M., Herberhold, C., and Elliott, M.J.. Successful complete tracheal resection in a three-month-old infant. Ann Thorac Surg 61,1824, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Rose, K.G., Sesterhenn, K., and Wustrow, F.. Tracheal allotransplantation in man. Lancet 1,433, 1979 [DOI] [PubMed] [Google Scholar]
- 50.Delaere, P.R., and Hermans, R.. Tracheal autotransplantation as a new and reliable technique for the functional treatment of advanced laryngeal cancer. Laryngoscope 113,1244, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Delaere, P., Vranckx, J., Verleden, G., De Leyn, P., Van Raemdonck, D., and Grp, L.T.T.. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. New Engl J Med 362,138, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Delaere, P.R., Vranckx, J.J., Meulemans, J., et al. Learning curve in tracheal allotransplantation. Am J Transplant 12,2538, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Delaere, P.R., Vranckx, J.J., Den Hondt, M., and Grp, L.T.T.. Tracheal allograft after withdrawal of immunosuppressive therapy. N Engl J Med 370,1568, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Delaere, P., and Van Raemdonck, D.. Tracheal replacement. J Thorac Dis 8,S186, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loos, E., Meulemans, J., Vranckx, J., Poorten, V.V., and Delaere, P.. Tracheal autotransplantation for functional reconstruction of extended hemilaryngectomy defects: a single-center experience in 30 patients. Ann Surg Oncol 23,1674, 2016 [DOI] [PubMed] [Google Scholar]
- 56.Klepetko, W., Marta, G.M., Wisser, W., et al. Heterotopic tracheal transplantation with omentum wrapping in the abdominal position preserves functional and structural integrity of a human tracheal allograft. J Thorac Cardiovasc Surg 127,862, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Olias, J., Millan, G., and da Costa, D.. Circumferential tracheal reconstruction for the functional treatment of airway compromise. Laryngoscope 115,159, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Hoffman, T.M., Gaynor, J.W., Bridges, N.D., Paridon, S.M., and Spray, T.L.. Aortic homograft interposition for management of complete tracheal anastomotic disruption after heart-lung transplantation. J Thorac Cardiovasc Surg 121,587, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Davidson, M.B., Mustafa, K., and Girdwood, R.W.. Tracheal replacement with an aortic homograft. Ann Thorac Surg 88,1006, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Wurtz, A., Porte, H., Conti, M., et al. Surgical technique and results of tracheal and carinal replacement with aortic allografts for salivary gland-type carcinoma. J Thorac Cardiovasc Surg 140,387, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Martinod, E., Paquet, J., Dutau, H., et al. In vivo tissue engineering of human airways. Ann Thorac Surg 103,1631, 2017 [DOI] [PubMed] [Google Scholar]
- 62.Azorin, J.F., Bertin, F., Martinod, E., and Laskar, M.. Tracheal replacement with an aortic autograft. Eur J Cardiothorac Surg 29,261, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Martinod, E., Radu, D.M., Chouahnia, K., et al. Human transplantation of a biologic airway substitute in conservative lung cancer surgery. Ann Thorac Surg 91,837, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Gilbert, T.W., Sellaro, T.L., and Badylak, S.F.. Decellularization of tissues and organs. Biomaterials 27,3675, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Elliott, M.J., De Coppi, P., Speggiorin, S., et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 380,994, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elliott, M.J., Butler, C.R., Varanou-Jenkins, A., et al. Tracheal replacement therapy with a stem cell-seeded graft: lessons from compassionate use application of a GMP-compliant tissue-engineered medicine. Stem Cells Transl Med 6,1458, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamilton, N.J., Kanani, M., Roebuck, D.J., et al. Tissue-engineered tracheal replacement in a child: a 4-year follow-up study. Am J Transplant 15,2750, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haykal, S., Soleas, J.P., Salna, M., Hofer, S.O., and Waddell, T.K.. Evaluation of the structural integrity and extracellular matrix components of tracheal allografts following cyclical decellularization techniques: comparison of three protocols. Tissue Eng Part C Methods 18,614, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Partington, L., Mordan, N.J., Mason, C., et al. Biochemical changes caused by decellularization may compromise mechanical integrity of tracheal scaffolds. Acta Biomater 9,5251, 2013 [DOI] [PubMed] [Google Scholar]
- 70.The Lancet. The final verdict on Paolo Macchiarini: guilty of misconduct. Lancet 392,2, 2018 [DOI] [PubMed] [Google Scholar]
- 71.Fux, T., Osterholm, C., Themudo, R., Simonson, O., Grinnemo, K.H., and Corbascio, M.. Synthetic tracheal grafts seeded with bone marrow cells fail to generate functional tracheae: first long-term follow-up study. J Thorac Cardiovasc Surg 159,2525, 2020 [DOI] [PubMed] [Google Scholar]
- 72.Kergin, F.G.Carcinoma of the trachea. J Thorac Surg 23,164, 1952 [PubMed] [Google Scholar]
- 73.Lee, J.Y., Park, J.H., and Cho, D.W.. Comparison of tracheal reconstruction with allograft, fresh xenograft and artificial trachea scaffold in a rabbit model. J Artif Organs 21,325, 2018 [DOI] [PubMed] [Google Scholar]
- 74.Bhora, F.Y., Lewis, E.E., Rehmani, S.S., et al. Circumferential three-dimensional-printed tracheal grafts: research model feasibility and early results. Ann Thorac Surg 104,958, 2017 [DOI] [PubMed] [Google Scholar]
- 75.Pepper, V., Best, C.A., Buckley, K., et al. Factors influencing poor outcomes in synthetic tissue-engineered tracheal replacement. Otolaryngol Head Neck Surg 161,458, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao, L.P., Sundaram, S., Le, A.V., et al. Engineered tissue-stent biocomposites as tracheal replacements. Tissue Eng Part A 22,1086, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park, J.H., Hong, J.M., Ju, Y.M., et al. A novel tissue-engineered trachea with a mechanical behavior similar to native trachea. Biomaterials 62,106, 2015 [DOI] [PubMed] [Google Scholar]
- 78.Ahn, C.B., Son, K.H., Yu, Y.S., Kim, T.H., Lee, J.I., and Lee, J.W.. Development of a flexible 3D printed scaffold with a cell-adhesive surface for artificial trachea. Biomed Mater 14,055001, 2019 [DOI] [PubMed] [Google Scholar]
- 79.Gao, M.C., Zhang, H.Y., Dong, W., et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci Rep 7,5246, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Best, C.A., Pepper, V.K., Ohst, D., et al. Designing a tissue-engineered tracheal scaffold for preclinical evaluation. Int J Pediatr Otorhinolaryngol 104,155, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sakaguchi, Y., Sato, T., Muranishi, Y., et al. Development of a novel tissue-engineered nitinol frame artificial trachea with native-like physical characteristics. J Thorac Cardiovasc Surg 156,1264, 2018 [DOI] [PubMed] [Google Scholar]
- 82.Taniguchi, D., Matsumoto, K., Tsuchiya, T., et al. Scaffold-free trachea regeneration by tissue engineering with bio-3D printing. Interact Cardiovasc Thorac Surg 26,745, 2018 [DOI] [PubMed] [Google Scholar]
- 83.Okuyama, H., Ohnishi, H., Nakamura, R., et al. Transplantation of multiciliated airway cells derived from human iPS cells using an artificial tracheal patch into rat trachea. J Tissue Eng Regen Med 13,1019, 2019 [DOI] [PubMed] [Google Scholar]
- 84.Nakaegawa, Y., Nakamura, R., Tada, Y., et al. Effects of artificial tracheal fixation on tracheal epithelial regeneration and prevention of tracheal stenosis. Acta Otolaryngol 137,627, 2017 [DOI] [PubMed] [Google Scholar]
- 85.Nakaegawa, Y., Nakamura, R., Tada, Y., et al. Effect of structural differences in collagen sponge scaffolds on tracheal epithelium regeneration. Ann Otol Rhinol Laryngol 125,115, 2016 [DOI] [PubMed] [Google Scholar]
- 86.Dahl, S.L., Kypson, A.P., Lawson, J.H., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 3,68ra9, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Kirkton, R.D., Santiago-Maysonet, M., Lawsonl, J.H., et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med 11,eaau6934, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Omori, K., Nakamura, T., Kanemaru, S., et al. Regenerative medicine of the trachea: the first human case. Ann Otol Rhinol Laryngol 114,429, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Omori, K., Tada, Y., Suzuki, T., et al. Clinical application of in situ tissue engineering using a scaffolding technique for reconstruction of the larynx and trachea. Ann Otol Rhinol Laryngol 117,673, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Grillo, H.C.Tracheal replacement: a critical review. Ann Thorac Surg 73,1995, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.