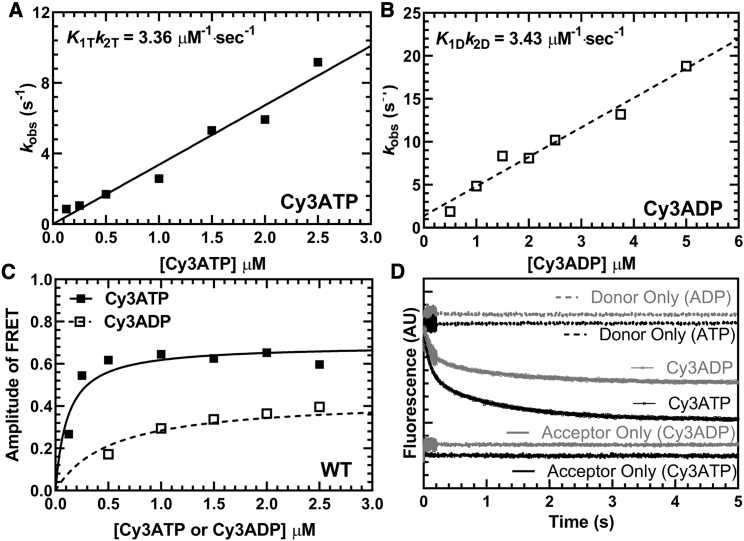
Figure 1.
Cy3-labeled nucleotide binding to M2β-S1. The FRET change observed upon Cy3ATP or Cy3ADP binding to M2β-S1 was monitored by mixing 1 μM M2β-S1 A488RLC with varying concentrations of fluorescent nucleotide and monitoring the decrease in donor fluorescence. Fluorescence transients were best fitted by a double-exponential function. (A) The observed fast phase rate constant for Cy3ATP binding to M2β-S1 was linearly dependent on Cy3ATP concentration, which allowed determination of the second-order binding constant. (B) Similarly, the observed fast phase rate constant for Cy3ADP binding to M2β-S1 was also linearly dependent on Cy3ADP concentration. (C) The amplitude of the FRET signal in the Cy3ATP and Cy3ADP experiments was plotted as a function of nucleotide concentration and was fitted to a hyperbolic function to compare the relative amplitudes of the FRET signal (Cy3ATP, AMax = 0.69 ± 0.04; Cy3ADP, AMax = 0.43 ± 0.04). (D) Representative fluorescent transients from the Cy3ATP and Cy3ADP binding experiments fitted to a double-exponential function (Cy3ATP, kFast = 8.8 ± 0.1 s−1, AFast = 0.52; Cy3ADP, kFast = 8.4 ± 0.1 s−1, AFast = 0.54).