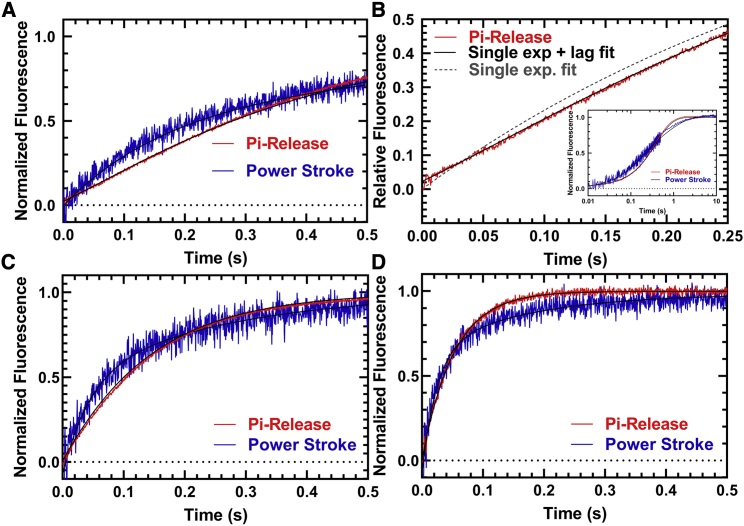
Figure 5.
Temperature dependence of actin-activated power stroke and phosphate release in M2β-S1. The phosphate binding protein (MDCC-PBP) was used to monitor the phosphate release step using sequential-mix experiments similar to that described in Fig. 4. 1–2 μM M2β-S1 A488RLC was mixed with substoichiometric ATP, aged for 10 s, and then mixed with 30 μM actin and MDCC-PBP. (A) Representative fluorescence transients (average of two to three normalized transients) of the power stroke (blue) and Pi release (red) in the presence of 30 μM actin are shown at 25°C. (B) A comparison of the Pi release transient (first 0.25 s) fitted to a single exponential function with (χ2 = 0.12) and without (χ2 = 0.29) a lag demonstrates an improved fit with the lag. The inset demonstrates the fit to the entire time course. A comparison of the power stroke and Pi release transients measured at (C) 30°C and (D) 35°C is given. The power-stroke experiments were best fitted to a double-exponential function at all temperatures. The Pi release experiments were best fitted by a lag followed by a single exponential fluorescence increase at 25 and 30°C, whereas the transients were single exponential at 35°C. (25°C, kFast = 7.3 ± 0.3 s−1, kSlow = 1.3 ± 0.1 s−1, kLag = 8.2 ± 0.7 s−1, kPi = 3.0 ± 0.1 s−1, AFast = 0.5; 30°C, kFast = 22 ± 1.4 s−1, kSlow = 3.7 ± 0.2 s−1, kLag = 21.6 ± 6.2 s−1, kPi = 7.1 ± 0.1 s−1, AFast = 0.5; 35°C, kFast = 36.5 ± 1.3 s−1, kSlow = 5.0 ± 0.2 s−1, no lag, kPi = 19.2 ± 0.1 s−1, AFast = 0.7).