Figure 6.
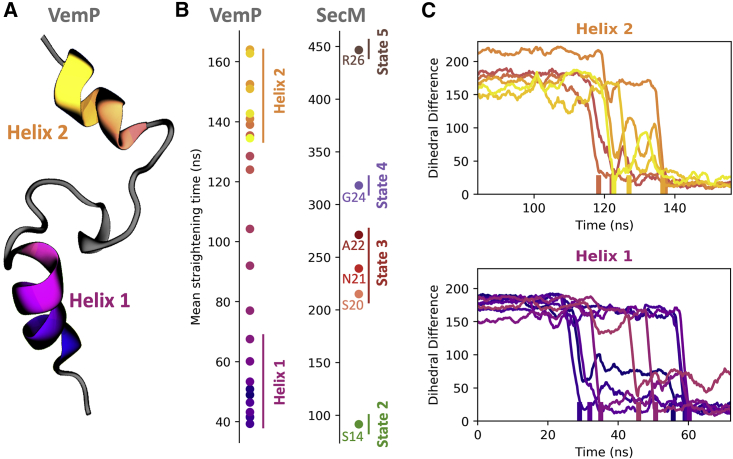
Force-induced conformational changes in VemP. (A) The structure of the stalled VemP nascent chain, with the two helical regions colored, is shown. (B) The straightening time for different amino acids in VemP and SecM averaged over 30 trajectories, using a force loading rate of 35 pN/ns for VemP and 50 pN/ns for SecM, is shown. (C) A time series of the deviation of the dihedral angles in helices 1 and 2 from the fully extended conformation, taken from a representative trajectory, is shown. The amino acids within a helix do not straighten in consecutive order. To see this figure in color, go online.