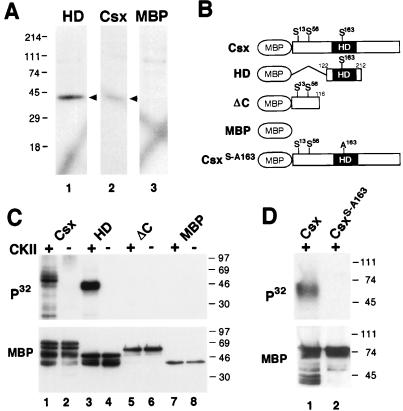
FIG. 4.
Phosphorylation of Csx/Nkx2.5 by CKII within the homeodomain. (A) In-gel kinase assays. NIH 3T3 cell nuclear extracts (25 μg) were separated in a gel containing either the homeodomain-MBP fusion protein (HD), the full-length Csx/Nkx2.5-MBP fusion protein (Csx), or MBP. After denaturation and renaturation, gels were incubated in kinase buffer containing [γ-32P]ATP. One kinase with a molecular mass of ∼40 kDa (arrowheads) phosphorylated the Csx/Nkx2.5 homeodomain, Csx/Nkx2.5 (lane 1), and the full-length Csx/Nkx2.5 (lane 2), but not MBP (lane 3). (B) Schematic representation of Csx/Nkx2.5 mutants carrying the consensus CKII sites and serine-to-alanine substitutions. (C) In vitro CKII phosphorylation of Csx/Nkx2.5. Three different Csx/Nkx2.5 fusion proteins described in panel B were incubated with [γ-32P]ATP in the presence (+) or absence (−) of purified CKII. Each sample was subjected to SDS-PAGE and transferred to a PVDF membrane. Autoradiography (upper panel) revealed that CKII phosphorylated the full-length Csx/Nkx2.5 (lane 1) and the homeodomain (lane 3) but neither the C-terminally deleted mutant (lane 5) nor MBP (lane 7). Loaded fusion proteins are shown by Western blotting of the same PVDF membrane using anti-MBP antibody (lower panel). (D) Serine 163 was mutated into alanine (Csx/Nkx2.5163S-A) and the kinase assay was performed. Lane 1 contains the wild-type Csx/Nkx2.5 and lane 2 contains the mutant Csx/Nkx2.5163S-A. Equal amounts of proteins were subjected to the kinase reaction as shown in the MBP Western blot (lower panel).