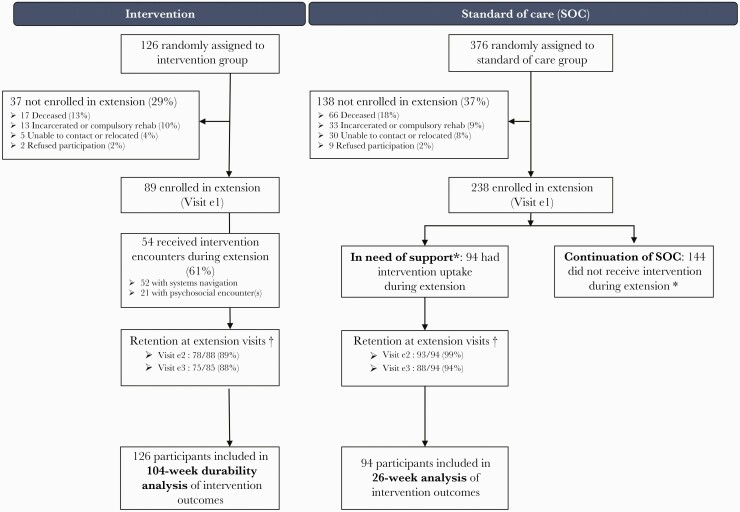
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. Enrollment and reenrollment participation, with primary trial intervention group on left and standard of care (SOC) group on right. *Participants originally randomized to SOC were recommended to receive intervention during the extension if they reported being off antiretroviral therapy and/or off medication for opioid use disorder at their last main study follow-up before the extension, or by site personnel discretion during the extension. †Retention denominator excludes participants who were deceased and nonrequired visits where the study closed before the participant’s targeted visit date. The denominator includes participants who missed the visit or exited study prematurely due to incarceration or compulsory rehabilitation, refusal to participate, unable to contact, or participant relocation. Extension visit e1 was the time of enrollment in the extension. Visit e1 is the extension enrollment. Visit e2 is extension week 13. Visit e3 is extension week 26.