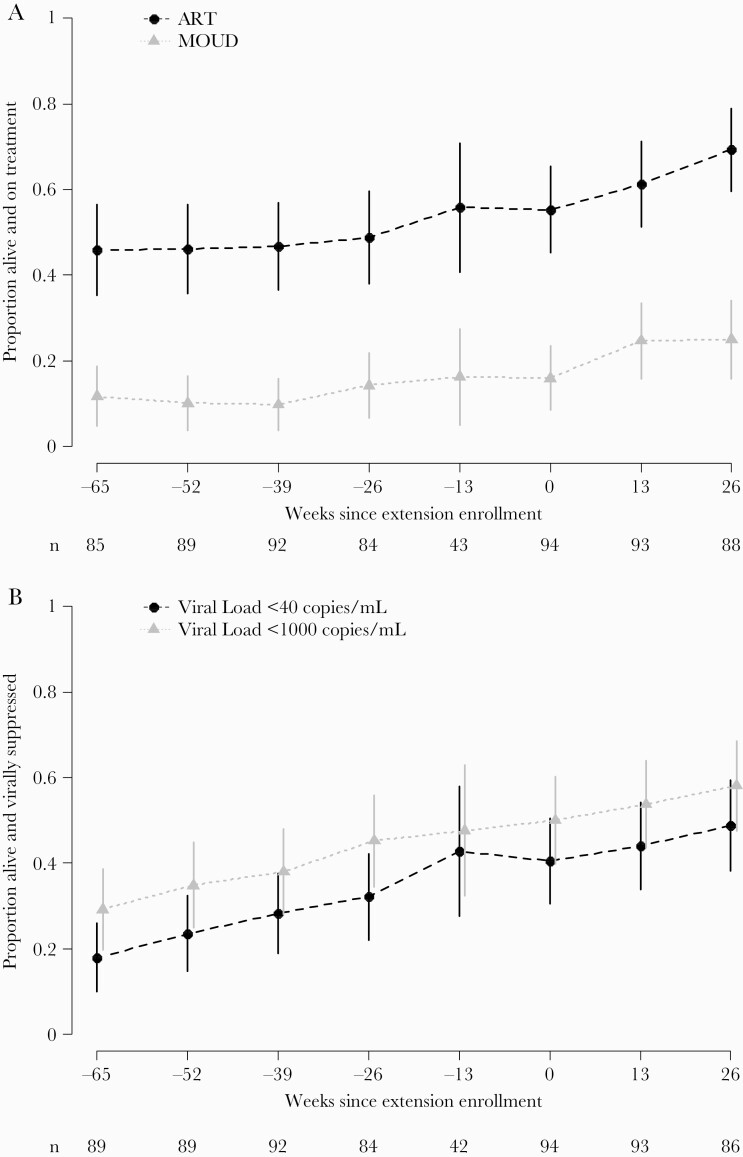
Figure 3.
Proportion of in need of support participants on treatment (A) and virally suppressed (B) by study week (n = 94). Standard of care (SOC) randomization arm participants who enrolled in the extension and received the intervention during the extension. Weeks since extension enrollment is displayed on the x-axis, and week –65 to week –13 outcomes occurred during the primary SOC study period before the extension. The proportion of participants who were alive and on treatment is shown on the y-axis with a corresponding 95% confidence interval at each follow-up. By definition, participants were alive at the time of their extension enrollment (week 0) and typically, these participants were off antiretroviral therapy (ART) or off medication for opioid use disorder (MOUD) at extension enrollment. The sample size at the visit prior to the extension (–13 weeks) reflects the time gap between the end of the primary study and the opening of extension enrollment.