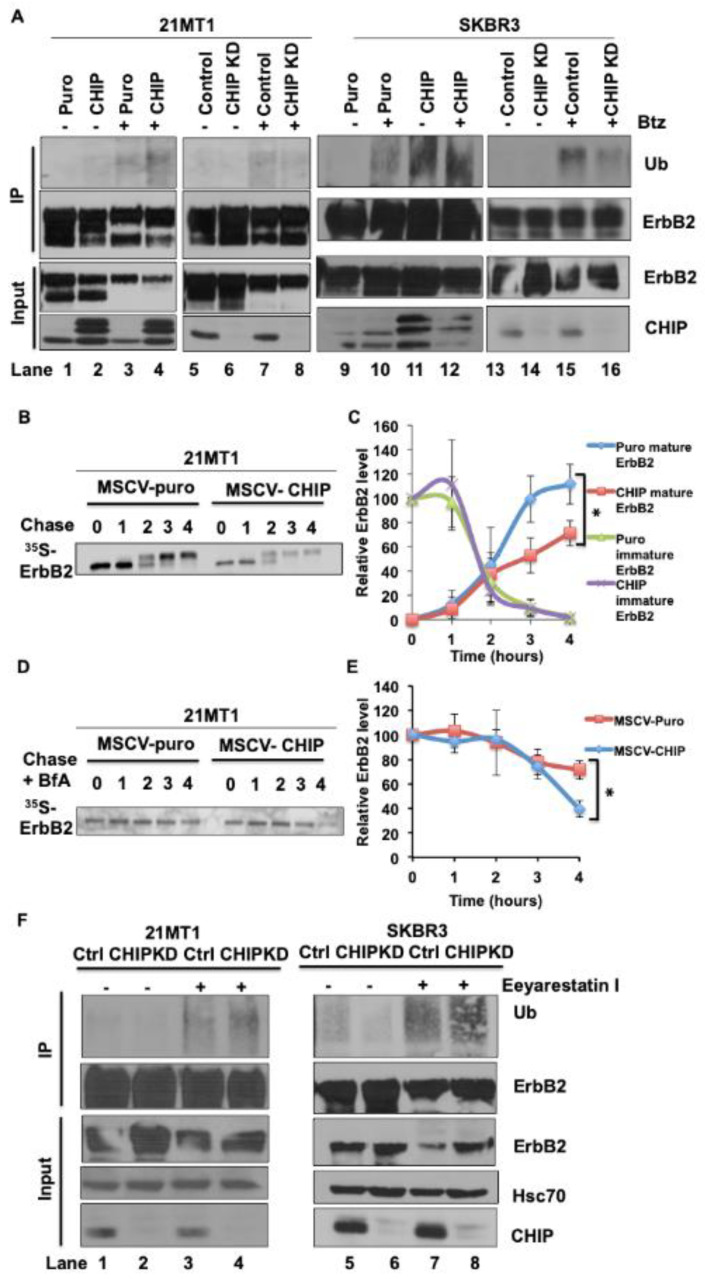
Figure 2.
CHIP elevates the basal ubiquitination of ErbB2 during its maturation. (A) 21MT1 and SKBR3 cells were seeded in 10 cm dishes and incubated with or without proteasome inhibitor Bortezomib (1 μM) for 4 h. Cleared lysates (1 mg protein) of the indicated cells were subjected to ErbB2 immunoprecipitation using Trastuzumab (5 μg/mL). Ubiquitinated ErbB2 signals were detected by anti-ubiquitin immunoblotting (upper panel). ErbB2 and CHIP levels in whole cell lysates are shown in the lower panel. (B) 21MT1 cells were pulse-labeled for 20 min with [35S]-methionine/cysteine and were then chased with excess (100-fold) unlabeled methionine/cysteine-containing medium. Cleared lysates from cells harvested at the indicated time points were used for immunoprecipitation with anti-ErbB2 antibody (Trastuzumab), and immunoprecipitates were resolved by SDS-PAGE and visualized by autoradiography. (C) Relative ErbB2 level quantification in B. Data represent mean ± SEM, n = 3, * p < 0.05. (D) 21MT1 cells were pulse-labeled for 20 min with [35S] methionine-cysteine and chased with excess unlabeled methionine-cysteine medium in the presence of Brefeldin A (1 μg/mL). Cleared lysates from cells harvested at the indicated time points were immunoprecipitated with anti-ErbB2 antibodies, followed by autoradiography. (E) Relative ErbB2 level quantification in (C). Data represent mean ± SEM, n = 3, * p < 0.05. (F) 21MT1 or SKBR3 cells were seeded in 10-cm dishes and incubated with or without Eeyarestatin I (1 μM) for 4 h. Cleared lysates (1 mg protein) of the indicated cells were subjected to ErbB2 immunoprecipitation using Trastuzumab (5 μg in 1 mL). Ubiquitinated ErbB2 signals were detected by anti-ubiquitin immunoblotting (upper panel). ErbB2 and CHIP levels in whole cell lysates are shown in the lower panel.