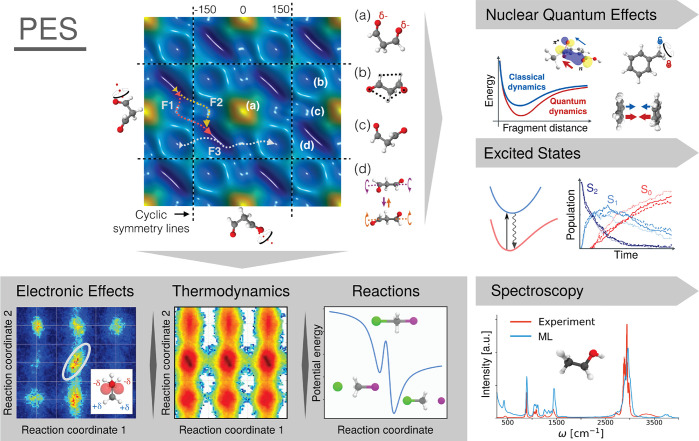
Figure 2.
ML-FFs combine the accuracy of ab initio methods and the efficiency of classical FFs. They provide easy access to a system’s potential energy surface (PES), which can in turn be used to derive a plethora of other quantities. By using them to run MD simulations on a single PES, ML-FFs allow chemical insights inaccessible to other methods (see gray box). For example, they accurately model electronic effects and their influence on thermodynamic observables and allow a natural description of chemical reactions, which is difficult or even impossible with conventional FFs. Their efficiency also allows them to be applied in situations where the Born–Oppenheimer approximation begins to break down and a single PES no longer provides an adequate description. An example is the study of nuclear quantum effects and electronically excited states (upper right). Finally, ML-FFs can be further enhanced by modeling additional properties. This provides direct access to a wide range of molecular spectra, building a bridge between theory and experiment (lower right). In general, such studies would be prohibitively expensive with ab initio methods.