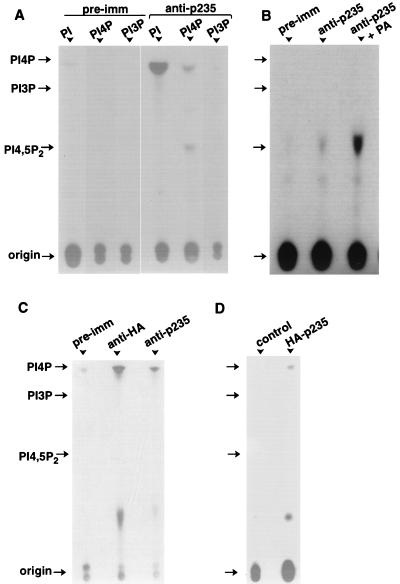
FIG. 11.
Lipid kinase activity of native and heterologously expressed p235. (A and B) Proteins from 3T3-L1 lysates were immunoprecipitated on preimmune (pre-imm) or anti-p235 peptide antibodies as described in Materials and Methods. After various washes, the immunoprecipitates were subjected to lipid kinase assay as described in Materials and Methods, in the presence of the indicated substrates (A) or PI 4-P (B). Where indicated, phosphatidic acid (PA; 140 μM; Avanti Polar Lipids Inc.) was present during the assay. (C) Lysates from COS-7 cells transfected with pCMV5-HA-p235 were immunoprecipitated with preimmune, anti-HA, or anti-p235 IgG as indicated. The immunoprecipitates were assayed for kinase activity as before with PI as a substrate. (D) COS-7 cells, transfected with pCMV5-HA-p235 or the empty vector, were immunoprecipitated with anti-HA IgG, and after SDS-PAGE the proteins were electrotransferred onto a nitrocellulose membrane. Transferred proteins were subjected to renaturation as described in Materials and Methods. Strips from both transfected and control lanes, corresponding to the electrophoretic mobility of HA-p235 (determined by Western blotting run in parallel) were excised and assayed for kinase activity in the presence of PI. Shown are TLC separations of extracted lipids and the positions of the indicated lipid standards and the origin (long arrow).