Figure 1.
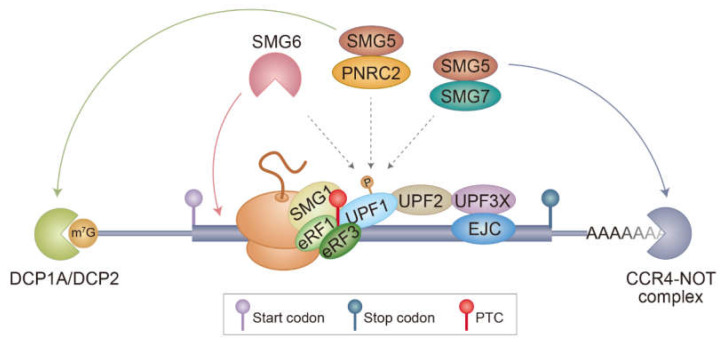
A model for canonical NMD. PTC is recognized during translation mediated by either CBC or eIF4E. A terminating ribosome on a PTC recruits SMG1 kinase, UPF1, and eRF1/3, forming the SURF complex. Association between the SURF complex and a PTC-distal EJC activates SMG1 kinase and triggers UPF1 hyperphosphorylation. The hyperphosphorylated UPF1 recruits several adaptors or ribonucleases, such as SMG5–SMG7 and PNRC2. Eventually, PTC-containing mRNAs are subjected to (I) endoribonucleolytic cleavage by SMG6, (II) decapping followed by 5’-to-3’ exoribonucleolytic degradation by PNRC2 and SMG5, and (III) deadenylation followed by 3’-to-5’ degradation by SMG5 and SMG7. Notably, the rapid degradation of NMD substrates may require all three mRNA decay pathways to act simultaneously or, alternatively, through one or two predominant pathways. The detailed molecular mechanisms underlying the choice between the above options need to be addressed by future studies.