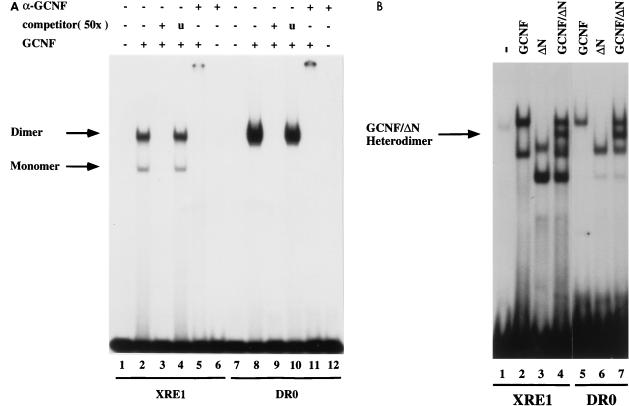
FIG. 2.
Binding of mGCNF to the XRE1 and the DR0. (A) Equal amounts of in vitro-translated mGCNF were assayed for binding to the XRE1 (lanes 2 to 5) and the DR0 (lanes 8 to 11) in EMSA. Unprimed reticulocyte lysate served as a control (lanes 1 and 7). Apparent monomeric or homodimeric mGCNF-DNA complexes are marked. DNA binding of mGCNF was competed with the XRE1 (lane 3), the DR0 (lane 9), or a control oligonucleotide containing an unrelated RZRβ binding site (lanes 4 and 10). DNA-bound mGCNF can be upshifted with a mGCNF-specific antibody (lanes 5 and 11). Lanes 6 and 12 contain only the antibody. (B) Heterodimer formation between mGCNF and ΔN-mGCNF on the XRE1 (lanes 2 to 4) and the DR0 (lanes 5 to 7). The position of the DNA-bound mGCNF–ΔN-mGCNF complex (lanes 4 and 7) is indicated by an arrow.