FIG. 4.
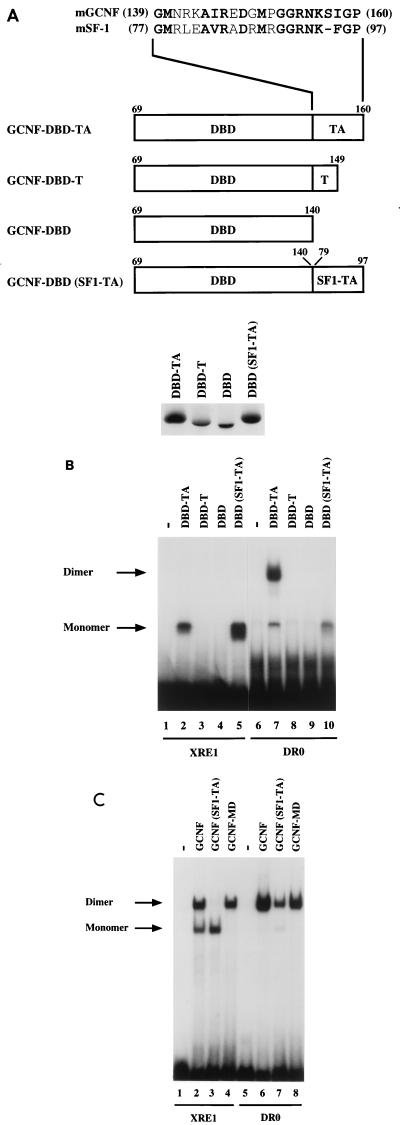
The TA box contributes to homodimeric DNA binding of mGCNF. (A) Schematic representation of mGCNF deletion and swap mutants. Numbers indicate the first and the last amino acids of mGCNF or mSF-1. The alignment shows the amino acid sequence of the TA box of mGCNF and mSF-1. All mutants were in vitro translated in similar amounts in reticulocyte lysate in the presence of [35S]methionine. Note that the mutants DBD-TA and DBD(SF1-TA) contain five methionine residues, whereas DBD-T and DBD contain only four and three methionine residues, respectively. (B) Binding of in vitro-translated mGCNF mutants to the XRE1 (lanes 2 to 5) and the DR0 (lanes 7 to 10) in EMSA. Unprimed reticulocyte lysate served as a control (lanes 1 and 6). (C) Binding of in vitro-translated mGCNF, mGCNF(SF1-TA), and mGCNF-MD to the XRE1 (lanes 2 to 4) and the DR0 (lanes 6 to 8) in EMSA. In mGCNF(SF1-TA), the TA box of mGCNF (amino acids 139 to 160) was replaced with the TA box of mSF-1 (amino acids 77 to 97). mGCNF-MD contains two point mutations (R113E and D114L) in the D box. Unprimed reticulocyte lysate served as a control in lanes 1 and 5.