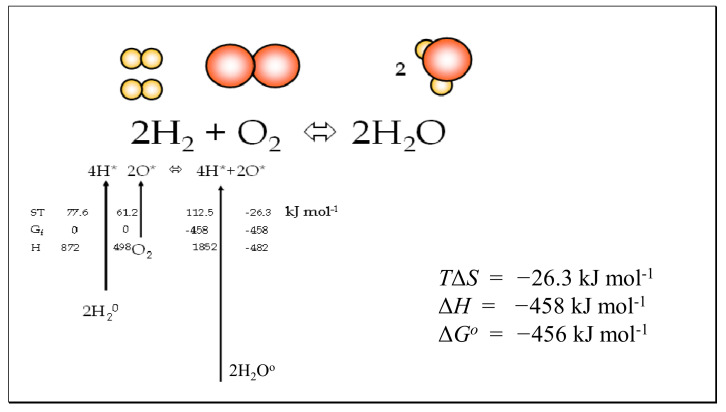
Figure 6.
Standard Gibbs energy change for the steady-state reaction forming water from hydrogen and oxygen. Potential changes are usually estimated using standard tables or more simply from the differences between cumulative bond energies and entropic energies using action mechanics. Bond energies at 298.15 K for HH, OO, and H2O are 436, 498, and 926 kJ per mole, respectively. Given ΔGo is −456 kJ mol−1, then lnK is 183.8 and K = 1079.8, indicating the forward reaction is hugely faster than the reverse reaction.