Abstract
Background:
In the phase III, 52-week ETHOS study in patients with moderate to very severe chronic obstructive pulmonary disease (COPD), triple therapy with budesonide/glycopyrrolate/formoterol fumarate (BGF), at two inhaled corticosteroid dose levels, resulted in significantly lower moderate/severe exacerbation rates versus glycopyrrolate/formoterol fumarate (GFF) and budesonide/formoterol fumarate (BFF). Here, we report results from the ETHOS pulmonary function test (PFT) sub-study, which assessed lung function in a subset of ETHOS patients.
Methods:
ETHOS (NCT02465567) was a randomized, double-blind, multi-center, parallel-group study in patients with moderate to very severe COPD who had experienced ⩾1 moderate/severe exacerbation in the previous year. Patients received BGF 320/18/9.6 µg, BGF 160/18/9.6 μg, GFF 18/9.6 µg, or BFF 320/9.6 µg twice daily via a single metered dose Aerosphere inhaler for 52 weeks. A subset of patients participated in the 4-hour PFT sub-study; primary endpoints were change from baseline in morning pre-dose trough forced expiratory volume in one second (FEV1) versus GFF and FEV1 area under the curve from 0 to 4 hours (AUC0–4) versus BFF at week 24.
Results:
The PFT modified intent-to-treat population included 3088 patients (mean age 64.4 years; mean reversibility post-albuterol 16.7%; mean post-albuterol FEV1% predicted 42.8). BGF 320/18/9.6 µg and 160/18/9.6 µg significantly improved morning pre-dose trough FEV1 at week 24 versus GFF (p ⩽ 0.0035 for both). Improvements in trough FEV1 were also observed at week 52 for BGF 320/18/9.6 µg and 160/18/9.6 µg versus GFF (p ⩽ 0.0005 for both). For FEV1 AUC0–4 at week 24, BGF 320/18/9.6 µg and 160/18/9.6 µg showed significant improvements versus BFF (p < 0.0001 for both). Improvements were maintained at week 52 (p < 0.0001).
Conclusions:
BGF 320/18/9.6 µg and 160/18/9.6 µg significantly improved trough FEV1 versus GFF and FEV1 AUC0–4 versus BFF at week 24. The lung function benefits with both doses of BGF were maintained following 52 weeks of treatment.
The reviews of this paper are available via the supplemental material section.
Keywords: BGF metered dose inhaler, chronic obstructive pulmonary disease, inhaled corticosteroid/long-acting muscarinic antagonist/long-acting β2-agonist (ICS/LAMA/LABA), pulmonary function, triple therapy
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation and is ranked as the third leading cause of mortality worldwide.1–3 In 2017, COPD had a global prevalence of approximately 300 million cases,4 was associated with approximately 3.2 million deaths,5 and was ranked as the seventh leading cause of disability worldwide.5
The use of inhaled corticosteroid/long-acting muscarinic antagonist/long-acting β2-agonist (ICS/LAMA/LABA) triple therapy is recommended for patients with COPD who experience persistent exacerbations, defined as an acute worsening of respiratory symptoms resulting in the need for additional therapy, or symptoms despite the use of dual LAMA/LABA, or ICS/LABA inhaled therapies.3 In such patients, triple therapies have been shown to improve lung function and symptoms and reduce the frequency of COPD exacerbations relative to dual combination therapies.3
In the ETHOS study (NCT02465567), the efficacy and safety of the ICS/LAMA/LABA triple fixed-dose combination therapy budesonide/glycopyrrolate/formoterol fumarate (BGF), at two ICS dose levels, delivered twice daily via a single metered dose Aerosphere inhaler, was assessed over a 52-week treatment period in symptomatic patients with moderate to very severe COPD, who had experienced at least one moderate or severe exacerbation in the previous 12 months. Moderate exacerbations were defined as those treated with corticosteroids with or without an antibiotic, and severe exacerbations were defined as those resulting in hospitalization or death.6 Treatment with BGF showed a significant reduction in the rate of moderate/severe COPD exacerbations, and improved symptoms and quality of life compared with glycopyrrolate/formoterol fumarate (GFF) and budesonide/formoterol fumarate (BFF).7 In addition, BGF 320/18/9.6 μg significantly reduced mortality versus GFF [hazard ratio 0.51, 95% confidence interval (CI) 0.33–0.80; unadjusted p-value 0.0035].8
A previous study of BGF, KRONOS, assessed symptomatic patients with moderate to very severe COPD without a requirement for a history of exacerbations (NCT02497001); primary endpoints were change from baseline in morning pre-dose trough forced expiratory volume in one second (FEV1) versus GFF and FEV1 area under the curve from 0 to 4 hours post-dose (AUC0–4) versus BFF at week 24. In the KRONOS study, BGF 320/18/9.6 μg significantly improved FEV1 AUC0–4 at week 24 compared with BFF; however, the improvement in the change from baseline in morning pre-dose trough FEV1 at week 24 for BGF 320/18/9.6 μg compared with GFF did not reach statistical significance.9
Here, we report data from the ETHOS pulmonary function test (PFT) sub-study, which assessed the effect of BGF relative to GFF and BFF on lung function, including the effect on the rate of decline, in a subset of patients in the ETHOS study, throughout the 52-week treatment period. In addition, we performed subgroup analyses based on FEV1 severity and blood eosinophil count at baseline.
Methods
Study design
Details of the primary ETHOS study design have been published.10,11 Briefly, ETHOS was a 52-week, randomized, double-blind, parallel-group trial conducted across 26 countries. Patients received twice daily dosing with BGF 320/18/9.6 µg, BGF 160/18/9.6 μg, GFF 18/9.6 µg, or BFF 320/9.6 µg. All treatments were administered via oral inhalation from a single metered dose Aerosphere inhaler; doses represent the sum of two actuations.
Eligible patients for the ETHOS study were 40–80 years of age, with symptomatic COPD (COPD assessment test score ⩾10 at screening despite receiving ⩾2 inhaled maintenance therapies), had a post-bronchodilator FEV1 25–65% of predicted normal, and had a smoking history of ⩾10 pack-years. If post-bronchodilator FEV1 was <50% of predicted normal, patients required a history of ⩾1 moderate or severe COPD exacerbation in the previous year, and if post-bronchodilator FEV1 was ⩾50% of predicted normal, a history of ⩾2 moderate or ⩾1 severe COPD exacerbations was required. Patients with a current diagnosis of asthma, respiratory disease other than COPD, or other significant uncontrolled diseases (including cardiac disease and cancer) were excluded.10,11
A subset of study sites was designated for participation in the PFT sub-study, which was conducted concurrently with the full study. For inclusion in the PFT sub-study, the average of the 60- and 30-minute pre-dose FEV1 assessments was required to be <65% predicted normal value at visit 4. In addition, patients were excluded from the PFT sub-study if they failed to meet American Thoracic Society/European Respiratory Society spirometry criteria for acceptability and repeatability.12 In order to ensure that baseline FEV1 values were stable and reflective of their true COPD severity during the screening period but prior to randomization, patients who did not meet FEV1 baseline stability criteria were also excluded; this was defined as the average of the 60- and 30-minute pre-dose FEV1 assessments at the randomization visit being within ±20% or 200 mL of the mean pre-dose FEV1 obtained at the two previous visits.
Lung function endpoints and assessments
Primary endpoints of the PFT sub-study included change from baseline in morning pre-dose trough FEV1 at week 24 and over 24 weeks for BGF versus GFF, and FEV1 AUC0–4 post-dose at week 24 and over 24 weeks for BGF versus BFF. Endpoints at week 24 were part of the US Food and Drug Administration (FDA) registration requirements and endpoints over 24 weeks were part of the European Medicines Agency (EMA) registration requirements. Other lung function endpoints included change from baseline in morning pre-dose trough FEV1 over 52 weeks, FEV1 AUC0–4 over 52 weeks, the onset of action (defined as the first time point at which the mean change from baseline in FEV1 exceeded 100 mL), and the rate of decline in pre-dose FEV1 and FEV1 AUC0–4 over 52 weeks. Baseline for all FEV1 analyses was calculated as the mean of 60- and 30-minute pre-dose FEV1 values obtained at randomization.
During the PFT sub-study, spirometry assessments were obtained at day 1 and weeks 4, 12, 24, 36, and 52. At these visits, spirometry assessments were conducted at 60 minutes and 30 minutes pre-dose and 5 (day 1 only), 15, and 30 minutes, and 1, 2, and 4 hours post-dose.
The FEV1 AUC0–4 was calculated using the trapezoidal rule, after first having subtracted the baseline FEV1 value, and the AUC was transformed into a weighted average by dividing by the time in hours from dosing to the last measurement included (typically 4 hours).
Statistical analyses
The PFT sub-study population was a subset of the patients in the modified intent-to-treat (mITT) population of the ETHOS study. The overall mITT population included all patients who were randomly assigned and treated and had post-randomization data obtained before discontinuation of treatment. The change from baseline in morning pre-dose trough FEV1 and differences between treatment groups in FEV1 AUC0–4 were analyzed using a repeated measures linear mixed model. The model included baseline FEV1, log baseline blood eosinophil count, and percentage reversibility to bronchodilator as continuous covariates, and visit, treatment, treatment by visit interaction, and ICS use at baseline as categorical covariates. Other endpoints were analyzed using a similar repeated measures linear mixed model such as morning pre-dose trough FEV1. The rates of decline in morning pre-dose trough FEV1 and FEV1 AUC0–4 were analyzed with a linear mixed model with random patient slopes of FEV1 versus time, and random patient intercepts. The rate of decline (the negative of the slope) was estimated and compared between treatments. The model included similar covariates to the analysis of trough FEV1 but with time in weeks as a continuous covariate in place of visit, smoking status as an additional categorical covariate and interactions between time and treatment, smoking status and baseline FEV1. Rate of decline analyses used changes from the week 4 visit. In addition, patients were stratified into subgroups based on post-bronchodilator FEV1 predicted (<50% and ⩾50%) and baseline blood eosinophil count (<150 cells/mm3 versus ⩾150 cells/mm3) to evaluate the potential impact of intrinsic or extrinsic factors on the results.
The primary endpoints were part of a type I error control procedure used for reporting the full ETHOS study. Other endpoints were not multiplicity controlled and were reported in terms of unadjusted p-values. Interpretation of results in subgroups relied on estimation and CIs.
In order to examine further the potential impact of lung function severity and eosinophil counts on lung function decline over one year, we performed an exploratory analysis of lung function decline in a pooled cohort of ICS-containing therapies (BGF 320/18/9.6 µg, BGF 160/18/9.6 μg, and BFF 320/9.6 µg) versus the non-ICS-containing therapy cohort (GFF 18/9.6 μg). As ICS may modulate lung function decline,13,14 the ICS-containing therapies were combined into a single group to reduce variability and increase overall sample size of the lung function severity and eosinophil subgroups.
Results
Study population
A total of 3088 patients were included in the 4-hour PFT sub-study (36.3% of the total ETHOS mITT population; mean age 64.4 years; mean reversibility post-albuterol 16.7%; mean post-albuterol FEV1% predicted, 42.8%; Table 1). Patient demographics were generally similar to those of the overall ETHOS mITT population.10
Table 1.
Demographics and baseline characteristics (PFT sub-study mITT population).
| BGF 320/18/9.6 µg n = 747 | BGF 160/18/9.6 µg n = 807 | GFF 18/9.6 µg n = 779 | BFF 320/9.6 µg n = 755 | |
|---|---|---|---|---|
| Age, years, mean (SD) | 64.3 (7.5) | 64.4 (7.6) | 64.8 (7.6) | 64.3 (7.5) |
| Male, n (%) | 397 (53.1) | 441 (54.6) | 386 (49.6) | 407 (53.9) |
| CAT score, mean (SD)a | 20.2 (6.5) | 20.5 (6.6) | 20.3 (6.8) | 20.4 (6.7) |
| Body mass index, kg/m2, mean (SD)b | 28.4 (6.6) | 28.3 (6.9) | 28.6 (6.6) | 27.9 (6.9) |
| Current smoker, n (%) | 331 (44.3) | 351 (43.5) | 336 (43.1) | 339 (44.9) |
| No. pack-years smoked,c median (range) | 44.0 (10.0–150.0) | 44.6 (10.0–187.5) | 43.0 (10.0–168.0) | 44.0 (10.0–250.0) |
| Baseline eosinophil count, n (%) | ||||
| <100 cells/mm3 | 106 (14.2) | 120 (14.9) | 95 (12.2) | 100 (13.2) |
| ⩾100 cells/mm3 | 641 (85.8) | 687 (85.1) | 684 (87.8) | 655 (86.8) |
| <150 cells/mm3 | 255 (34.1) | 303 (37.5) | 266 (34.1) | 247 (32.7) |
| ⩾150 cells/mm3 | 492 (65.9) | 504 (62.5) | 513 (65.9) | 508 (67.3) |
| Exacerbation history, n (%) | ||||
| 1 | 349 (46.7) | 371 (46.0) | 352 (45.2) | 343 (45.4) |
| ⩾2 | 398 (53.3) | 436 (54.0) | 427 (54.8) | 412 (54.6) |
| Post-albuterol FEV1% of predicted normal, mean (SD) | 43.1 (10.4) | 42.5 (10.4) | 43.0 (10.3) | 42.7 (10.5) |
| Reversibility post-albuterol FEV1,%, mean (SD)d | 17.4 (16.6) | 16.3 (16.3) | 17.3 (15.9) | 15.8 (15.4) |
| Reversible, n (%) | 264 (35.3) | 257 (31.8) | 280 (35.9) | 251 (33.2) |
| Use of ICS at screening, n (%) | 562 (75.2) | 628 (77.8) | 598 (76.8) | 573 (75.9) |
BGF 320/18/9.6 µg, n = 745; BGF 160/18/9.6 µg, n = 806.
BGF 160/18/9.6 µg, n = 806.
Number of pack-years smoked = (number of cigarettes per day/20) × number of years smoked.
BGF 320/18/9.6 µg, n = 745; BGF 160/18/9.6 µg, n = 806; GFF 18/9.6 µg, n = 778; BFF 320/9.6 µg, n = 753.
BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GFF, glycopyrrolate/formoterol fumarate; ICS, inhaled corticosteroid; mITT, modified intent-to-treat; PFT, pulmonary function test; SD, standard deviation.
Morning pre-dose trough FEV1
BGF 320/18/9.6 μg treatment resulted in significant improvements in least squares (LS) mean change from baseline morning pre-dose trough FEV1 at week 24 compared with GFF (35 mL; p-value 0.0025; Table 2) and BFF (76 mL; unadjusted p-value < 0.0001; Table 2). Treatment with BGF 160/18/9.6 μg also significantly improved LS mean change from baseline in morning pre-dose trough FEV1 at week 24 compared with GFF (33 mL; p-value 0.0035; Table 2) and BFF (74 mL; unadjusted p-value < 0.0001; Table 2).
Table 2.
Change from baselinea in morning pre-dose trough FEV1 and FEV1 AUC0–4 (efficacy estimand; PFT sub-study mITT populationb).
| BGF 320/18/9.6 μg versus GFF 18/9.6 μg | BGF 160/18/9.6 μg versus GFF 18/9.6 μg | BGF 320/18/9.6 μg versus BFF 320/9.6 μg | BGF 160/18/9.6 μg versus BFF 320/9.6 μg | ||
|---|---|---|---|---|---|
| Change from baseline in morning pre-dose trough FEV1, mLc | |||||
| At week 24 | LSM (95% CI) | 35 (12, 57) | 33 (11, 55) | 76 (54, 99) | 74 (52, 96) |
| p-value | 0.0025 | 0.0035 | <0.0001 | <0.0001 | |
| Over 24 weeks | LSM (95% CI) | 43 (25, 60) | 30 (12, 47) | 76 (58, 94) | 63 (46, 81) |
| p-value | <0.0001 | 0.0009 | <0.0001 | <0.0001 | |
| At week 52 | LSM (95% CI) | 55 (30, 79) | 43 (18, 67) | 65 (40, 89) | 53 (29, 77) |
| p-value | <0.0001 | 0.0005 | <0.0001 | <0.0001 | |
| Over 52 weeks | LSM (95% CI) | 46 (27, 64) | 36 (18, 54) | 72 (54, 90) | 62 (45, 80) |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| FEV1 AUC0–4, mLc | |||||
| At week 24 | LSM (95% CI) | 53 (29, 77) | 43 (19, 66) | 119 (95, 143) | 109 (85, 132) |
| p-value | <0.0001 | 0.0004 | <0.0001 | <0.0001 | |
| Over 24 weeks | LSM (95% CI) | 49 (31, 66) | 34 (17, 51) | 99 (82, 117) | 85 (67, 102) |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| At week 52 | LSM (95% CI) | 66 (40, 92) | 55 (30, 80) | 108 (82, 133) | 97 (71, 122) |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Over 52 weeks | LSM (95% CI) | 53 (35, 71) | 41 (23, 59) | 102 (84, 120) | 90 (72, 108) |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
Baseline was defined as the mean of the 30- and 60-minute values prior to dosing on day 1, if available; otherwise, the mean of the 30- and 60-minute pre-bronchodilator assessments at visit 3 were used, if available; otherwise, the mean of the 30- and 60-minute pre-bronchodilator assessments at visit 2 were used.
mITT population: BGF 320/18/9.6 μg, n = 747; BGF 160/18/9.6 μg, n = 807; GFF 18/9.6 μg, n = 779; BFF 320/9.6 μg, n = 755.
The pre-specified treatment comparisons of interest were both doses of BGF versus GFF (for trough FEV1), and both doses of BGF versus BFF (for FEV1 AUC0–4). Results in bold were type I error-controlled; all other comparisons were not adjusted for multiplicity.
AUC0–4, area under the curve from 0 to 4 hours post-dose; BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; CI, confidence interval; FEV1, forced expiratory volume in 1 second; GFF, glycopyrrolate/formoterol fumarate; LSM, least squares mean; mITT, modified intent-to-treat; PFT, pulmonary function test.
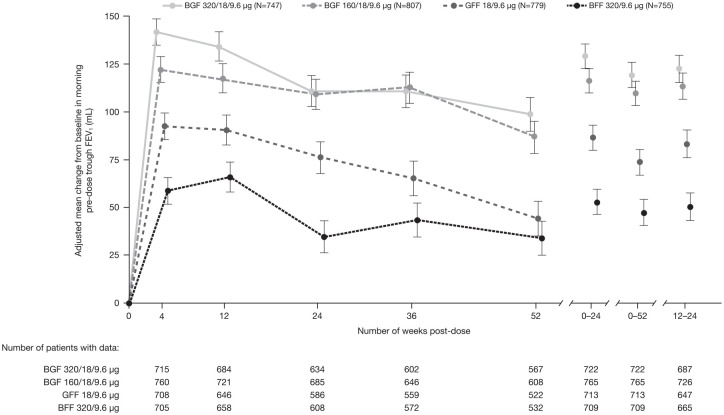
Significant improvements in morning pre-dose trough FEV1 were maintained at week 52 for BGF 320/18/9.6 µg and BGF 160/18/9.6 µg versus both GFF and BFF (unadjusted p-value ⩽ 0.0005; Table 2). Improvements in morning pre-dose trough FEV1 with both doses of BGF versus GFF and BFF were sustained throughout the 52-week treatment period (Figure 1). While there was no appreciable difference between BGF doses at week 24, there was a small numerical difference in favor of BGF 320/18/9.6 μg versus BGF 160/18/9.6 μg over 24 weeks (Figure 1; Table 2).
Figure 1.
Change from baseline in morning pre-dose trough FEV1 over study duration (efficacy estimand; PFT mITT sub-study population).
Data are adjusted mean ± standard error.
BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; FEV1, forced expiratory volume in 1 second; GFF, glycopyrrolate/formoterol fumarate; mITT, modified intent-to-treat; PFT, pulmonary function test.
FEV1 AUC0–4
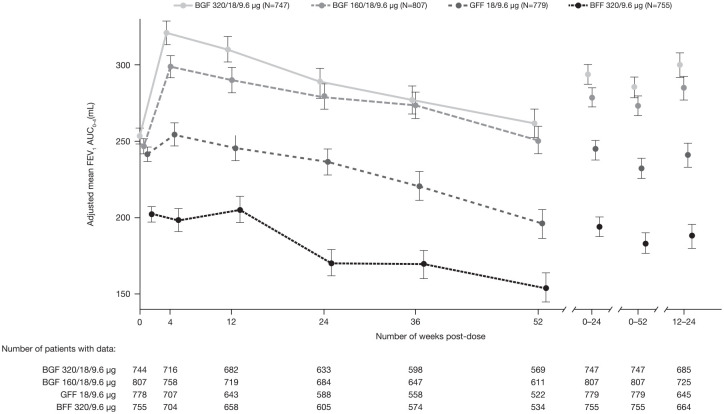
BGF 320/18/9.6 μg treatment resulted in significant improvements in LS mean FEV1 AUC0–4 at week 24 compared with BFF (119 mL; p-value < 0.0001; Table 2) and GFF (53 mL; unadjusted p-value < 0.0001; Table 2). Treatment with BGF 160/18/9.6 μg also significantly improved LS mean FEV1 AUC0–4 at week 24 compared with BFF (109 mL; p-value < 0.0001) and GFF (43 mL; unadjusted p-value 0.0004; Table 2). There were small numerical differences in favor of BGF 320/18/9.6 μg versus BGF 160/18/9.6 μg both at week 24 and over 24 weeks (Figure 2; Table 2).
Figure 2.
FEV1 AUC0–4 over study duration (efficacy estimand; PFT mITT sub-study population).
Data are adjusted mean ± standard error.
AUC0–4, area under the curve from 0 to 4 hours post-dose; BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; FEV1, forced expiratory volume in 1 second; GFF, glycopyrrolate/formoterol fumarate; mITT, modified intent-to-treat; PFT, pulmonary function test.
Significant improvements in FEV1 AUC0–4 were maintained at week 52 for BGF 320/18/9.6 μg and BGF 160/18/9.6 μg versus BFF and GFF (unadjusted p-value <0.0001 for all comparisons; Table 2). Improvements in FEV1 AUC0–4 with both doses of BGF versus BFF and GFF were sustained throughout the 52-week treatment period (Figure 2).
Analyses by FEV1% predicted at baseline (<50% versus ⩾50%)
The effects of BGF versus GFF and BFF on morning pre-dose trough FEV1 and FEV1 AUC0–4 at week 24 in patients with post-bronchodilator FEV1 < 50% and ⩾50% predicted were directionally consistent with the overall findings but with slightly larger estimated benefits in the subgroup with post-bronchodilator FEV1 ⩾ 50% (Table 3).
Table 3.
Change from baseline in morning pre-dose trough FEV1 and FEV1 AUC0–4 at week 24 by post-bronchodilator FEV1% predicted (efficacy estimand; PFT sub-study mITT population).
| % Predicted post-bronchodilator FEV1 | BGF 320/18/9.6 μg versus GFF 18/9.6 μg | BGF 160/18/9.6 μg versus GFF 18/9.6 μg | BGF 320/18/9.6 μg versus BFF 320/9.6 μg | BGF 160/18/9.6 μg versus BFF 320/9.6 μg | |
|---|---|---|---|---|---|
| Morning pre-dose trough FEV1, mL | |||||
| <50% n = 2250 | LSM (95% CI) | 31 (7, 55) | 29 (5, 53) | 71 (47, 95) | 69 (45, 92) |
| p-value | 0.0118 | 0.0180 | <0.0001 | <0.0001 | |
| ⩾50% n = 838 | LSM (95% CI) | 47 (−4, 97) | 47 (−2, 95) | 90 (39, 140) | 89 (40, 138) |
| p-value | 0.0705 | 0.0624 | 0.0006 | 0.0004 | |
| FEV1 AUC0–4, mL | |||||
| <50% n = 2250 | LSM (95% CI) | 48 (22, 75) | 39 (13, 65) | 112 (86, 138) | 103 (77, 129) |
| p-value | 0.0003 | 0.0036 | <0.0001 | <0.0001 | |
| ⩾50% n = 838 | LSM (95% CI) | 67 (16, 118) | 57 (8, 107) | 135 (83, 186) | 125 (75, 175) |
| p-value | 0.0108 | 0.0241 | <0.0001 | <0.0001 | |
AUC0–4, area under the curve from 0 to 4 hours post-dose; BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; CI, confidence interval; FEV1, forced expiratory volume in 1 second; GFF, glycopyrrolate/formoterol fumarate; LSM, least squares mean; mITT, modified intent-to-treat; PFT, pulmonary function test.
Analyses by baseline blood eosinophil count (<150 cells/mm3 versus ⩾150 cells/mm3)
Similarly, the effects of BGF versus GFF and BFF on morning pre-dose trough FEV1 and FEV1 AUC0–4 at week 24 in patients with blood eosinophil count <150 cells/mm3 and ⩾150 cells/mm3 were directionally consistent with the overall findings but with slightly larger estimated benefits in the subgroup with blood eosinophil count ⩾150 cells/mm3 (Table 4).
Table 4.
Change from baseline in morning pre-dose trough FEV1 and FEV1 AUC0–4 at week 24 by baseline blood eosinophil count (efficacy estimand; PFT sub-study mITT population).
| Baseline eosinophil count | BGF 320/18/9.6 μg versus GFF 18/9.6 μg | BGF 160/18/9.6 μg versus GFF 18/9.6 μg | BGF 320/18/9.6 μg versus BFF 320/9.6 μg | BGF 160/18/9.6 μg versus BFF 320/9.6 μg | |
|---|---|---|---|---|---|
| Morning pre-dose trough FEV1, mL | |||||
| <150 cells/mm3 n = 1071 | LSM (95% CI) | 12 (−24, 47) | −5 (−39, 29) | 71 (34, 107) | 54 (20, 89) |
| p-value | 0.5266 | 0.7828 | 0.0001 | 0.0022 | |
| ⩾150 cells/mm3 n = 2017 | LSM (95% CI) | 45 (17, 74) | 55 (26, 83) | 77 (49, 105) | 86 (58, 114) |
| p-value | 0.0019 | 0.0002 | <0.0001 | <0.0001 | |
| FEV1 AUC0–4, mL | |||||
| <150 cells/mm3 n = 1071 | LSM (95% CI) | 31 (−7, 68) | 2 (−34, 37) | 117 (79, 155) | 88 (52, 124) |
| p-value | 0.1063 | 0.9317 | <0.0001 | <0.0001 | |
| ⩾150 cells/mm3 n = 2017 | LSM (95% CI) | 64 (33, 94) | 68 (37, 98) | 120 (89, 150) | 123 (93, 153) |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
AUC0–4, area under the curve from 0 to 4 hours post-dose; BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; CI, confidence interval; FEV1, forced expiratory volume in 1 second; GFF, glycopyrrolate/formoterol fumarate; LSM, least squares mean; mITT, modified intent-to-treat; PFT, pulmonary function test.
The change from baseline in morning pre-dose trough FEV1 and FEV1 AUC0–4 at week 24 for both doses of BGF versus GFF and BFF in patients with blood eosinophil counts <100 cells/mm3, 100–<300 cells/mm3, and ⩾300 cells/mm3 is shown in Supplemental Table 1. Larger changes from baseline in morning pre-dose trough FEV1 and FEV1 AUC0–4 at week 24 were seen for both doses of BGF versus GFF from blood eosinophil counts ⩾100 cells/mm3, which increased as baseline blood eosinophil count increased. Benefits were seen in morning pre-dose trough FEV1 and FEV1 AUC0–4 at week 24 for both doses of BGF versus BFF, although these did not vary across eosinophil levels to the extent observed for BGF versus GFF.
Onset of action
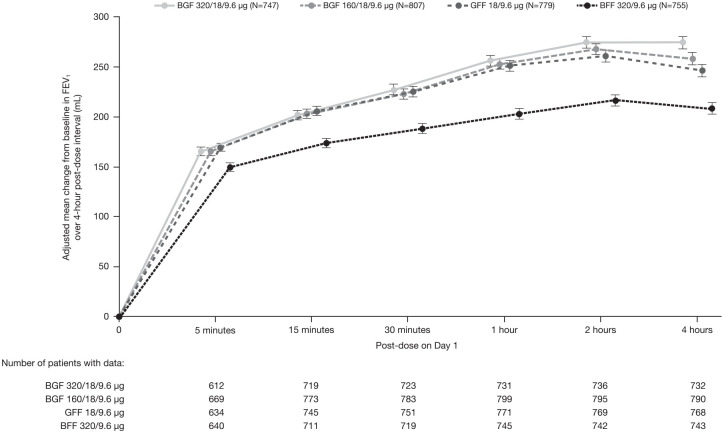
Improvements in FEV1 were achieved rapidly with post-dose changes from baseline in FEV1 being >100 mL for all four treatment groups at the 5-minute post-dose measurement (Figure 3).
Figure 3.
Change from baseline in FEV1 over 4-hour post-dose interval at day 1 (efficacy estimand; PFT mITT sub-study population).
Data are adjusted mean ± standard error.
BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; FEV1, forced expiratory volume in 1 second; GFF, glycopyrrolate/formoterol fumarate; mITT, modified intent-to-treat; PFT, pulmonary function test.
Analyses of ICS-containing therapies versus GFF on lung function decline
A trend for a lower rate of decline in morning pre-dose trough FEV1 over 52 weeks was observed in the BGF treatment groups relative to GFF (Supplemental Table 2). However, no consistent effects were observed for a lower rate of decline in FEV1 AUC0–4 for BGF relative to BFF (Supplemental Table 2).
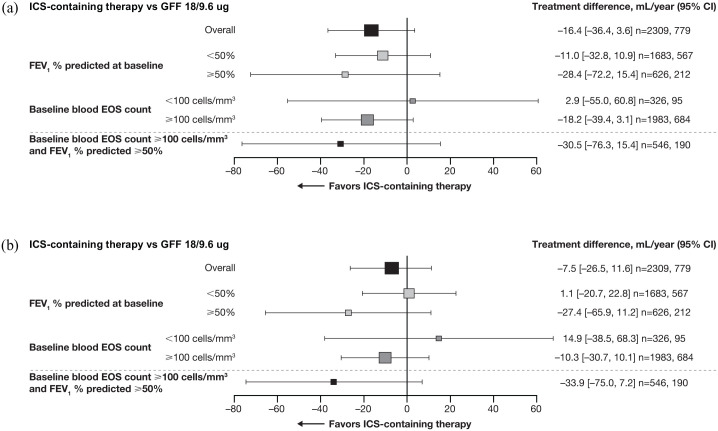
Patients treated with ICS-containing therapies were pooled to assess whether benefits versus the non-ICS-containing therapy, GFF, were driven by acute ICS withdrawal in patients who received ICS prior to randomization and to increase the sample size of the subgroup. Pooling patients treated with ICS-containing therapies showed lower annual rates of decline in pre-dose trough FEV1 versus GFF [treatment difference −16.4 mL (95% CI −36.4 mL, 3.6 mL); Table 5]. A smaller reduction in the annual rate of decline was also seen for FEV1 AUC0−4 in patients treated with ICS-containing therapies versus GFF [treatment difference −7.5 mL (95% CI −26.5 mL, 11.6 mL); Table 5]. Patients with moderate airflow obstruction (FEV1 ⩾50% predicted at baseline) and blood eosinophil counts ⩾100 cells/mm3 had a greater reduction in the adjusted annual rates of decline in morning pre-dose trough FEV1 and FEV1 AUC0–4 when treated with ICS-containing therapies versus GFF (Figure 4).
Table 5.
Adjusted rate of decline in morning pre-dose trough FEV1 and FEV1 AUC0–4 over 52 weeks by ICS-containing therapy (efficacy estimand; PFT sub-study mITT population).
| ICS-containing therapy N = 2309 | GFF 18/9.6 μg N = 779 | |
|---|---|---|
| Morning pre-dose FEV1, mL/yeara | ||
| Adjusted rate of decline (SE) | 37.7 (5.0) | 54.0 (8.9) |
| Treatment difference (95% CI) | – | −16.4 (−36.4, 3.6) |
| FEV1 AUC0–4, mL/yearb | ||
| Adjusted rate of decline (SE) | 56.1 (4.7) | 63.6 (8.5) |
| Treatment difference (95% CI) | – | −7.5 (−26.5, 11.6) |
Rate of the decline of pre-dose trough FEV1 is –1 multiplied by the average of the individual slope of pre-dose trough FEV1 over 52 weeks across patients for the treatment.
Rate of the decline of FEV1 AUC0–4 is –1 multiplied by the average of the individual slope of FEV1 AUC0–4 over 52 weeks across patients for the treatment.
AUC0–4, area under the curve from 0 to 4 hours post-dose; BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; CI, confidence interval; FEV1, forced expiratory volume in 1 second; GFF, glycopyrrolate/formoterol fumarate; ICS, inhaled corticosteroid; mITT, modified intent-to-treat; PFT, pulmonary function test; SE, standard error.
Figure 4.
Adjusted rate of decline in pre-dose trough FEV1 (a) and FEV1 AUC0–4 (b) over 52 weeks.
Size of data point relative to size of patient cohort; n are for ICS-containing therapies and GFF 18/9.6 ug, respectively.
AUC0–4, area under the curve 0 to 4 hours post-dose; CI, confidence interval; EOS, eosinophil; FEV1, forced expiratory volume in 1 second; GFF, glycopyrrolate/formoterol fumarate; ICS, inhaled corticosteroid.
Discussion
The findings of this 4-hour PFT sub-study of ETHOS demonstrated the benefit of BGF versus GFF and BFF on both morning pre-dose trough FEV1 and FEV1 AUC0–4 for the first 24 weeks of treatment. These improvements in lung function were sustained at week 52. These findings are in line with the recommendations in the GOLD report, which notes that triple therapy can improve lung function versus dual LAMA/LABA and ICS/LABA therapies.3 While ETHOS was the first study of a triple fixed-dose combination therapy to evaluate two different ICS doses in the same study, it was not designed or powered to detect significant differences between the doses of BGF. Numerical trends in favor of BGF 320/18/9.6 μg over BGF 160/18/9.6 μg relative to GFF were observed for the PFT sub-study primary endpoints; however, these differences were not large. The effects of the ICS component of BGF, as reflected by improvements in trough FEV1 with BGF versus GFF, were greatest in the subgroup of patients with FEV1 ⩾ 50% predicted and eosinophil counts ⩾100 cells/mm3.
The benefits of BGF 320/18/9.6 μg on lung function at week 24 observed in ETHOS are consistent with data from the KRONOS study, in which BGF demonstrated significant improvements in lung function relative to ICS/LABA dual therapy.9 As in ETHOS, a statistically significant improvement in FEV1 AUC0–4 at week 24 was also observed for BGF 320/18/9.6 μg compared with BFF in KRONOS. However, unlike ETHOS, the improvement in change from baseline in morning pre-dose trough FEV1 at week 24 compared with GFF in KRONOS was numerical, but did not achieve statistical significance.9 Nonetheless, it is important to note that analyses at week 24 with the attributable estimand in KRONOS, which factored in missing data, did demonstrate a significant difference between BGF 320/18/9.6 μg and GFF for trough FEV1 (24 mL; unadjusted p-value = 0.0370). This suggested that missing data played an important role in the trough FEV1 results for BGF 320/18/9.6 μg versus GFF in KRONOS.
As reported previously, lung function improvements with an ICS are known to be associated with blood eosinophil counts.15–20 In this regard, the percentage of patients with baseline blood eosinophil levels <150 cells/mm3 was 48.2% and 40.0% in the overall populations for KRONOS and ETHOS, respectively. The percentage of patients with blood eosinophil levels <150 cells/mm3 was considerably higher in KRONOS compared with ETHOS, which may explain the lower magnitude of benefit observed in pre-dose trough FEV1 for BGF relative to GFF at week 24 in KRONOS. Nonetheless, the improvements observed in morning pre-dose trough FEV1 at week 24 in ETHOS for BGF relative to GFF (35 mL) were consistent with values observed for the ICS component for this endpoint in other triple fixed-dose combinations at week 24 or week 26 (20 mL to 81 mL).15,18,21
Although the current study was not designed to evaluate lung function decline, a trend for a lower rate of decline in morning pre-dose trough FEV1 over 52 weeks was observed for both doses of BGF relative to GFF. No consistent effects were observed for rate of decline in FEV1 AUC0–4 over 52 weeks for BGF relative to BFF. However, exploratory analyses pooling ICS-containing therapies were also conducted to evaluate the effects of blood eosinophils and lung function severity on lung function decline, as these factors are known to modulate the lung function benefits of ICS in COPD. Results using the pooled data suggested that greater reductions in the annual rate of lung function decline relative to the LAMA/LABA occurred in patients with moderate airflow obstruction and those with a baseline blood eosinophil count ⩾100 cells/mm3. Although speculative, these findings suggest that there may be value in initiating ICS therapy to prevent lung function decline in COPD patients with eosinophil counts ⩾100 cells/mm3 and less severe lung function impairment, rather than waiting until a marked loss of lung function has already occurred. Clearly, prospective studies aimed at reducing the rate of lung function decline earlier in the COPD disease process are needed.
In conclusion, both BGF 320/18/9.6 μg and BGF 160/18/9.6 μg provided significant improvements in lung function at week 24 versus GFF and BFF in patients with moderate to very severe COPD. The benefits on lung function were sustained versus ICS/LABA and LAMA/LABA dual therapies over 52 weeks. The lung function improvements observed in this sub-study complement the improvements observed in exacerbations, symptoms, and quality of life in the overall ETHOS study in patients with moderate to very severe COPD.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-6-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors would like to thank all the patients, their families, and the investigators, research nurses, and operations staff involved in ETHOS. The authors also thank Julie McLaren, Earl St Rose, Shaila Ballal, and Colin Reisner, who were employees of AstraZeneca when this study was conducted, and held stock options in the company, for their valuable contribution to the study. Medical writing support, under the direction of the authors, was provided by Jake Casson, CMC Connect, McCann Health Medical Communications, and was funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines.22
Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC)
Footnotes
Author contributions: The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Klaus F Rabe, Fernando J Martinez, Dave Singh, Roopa Trivedi, and Magnus Aurivillius: Acquisition of data; data interpretation.
Martin Jenkins, Patrick Darken, and Paul Dorinsky: Conception/design; data analysis/interpretation.
Conflict of interest statement: Klaus F Rabe reports personal fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi Pharmaceuticals, InterMune, Novartis, Sanofi, and Teva; and grants from the Ministry of Education and Science, Germany, outside the submitted work.
Fernando J Martinez reports grants from AstraZeneca during the conduct of the study; personal fees and non-financial support from the American College of Chest Physicians, AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, Concert, Continuing Education, Genentech, GlaxoSmithKline, Inova Fairfax Health System, Miller Communications, the National Association for Continuing Education, Novartis, PeerView Communications, Prime Communications, the Puerto Rican Respiratory Society, Roche, Sunovion, and Theravance; non-financial support from ProterixBio; and personal fees from the American Thoracic Society, Columbia University, Haymarket Communications, Integritas, inThought Research, MD Magazine, Methodist Hospital Brooklyn, New York University, Unity, Up-To-Date, WebMD/MedScape, and Western Connecticut Health Network; and grants from the National Institutes of Health, outside the submitted work.
Dave Singh reports personal fees from Apellis, Cipla, Genentech, Peptinnovate, and Skyepharma; and grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, GlaxoSmithKline, Glenmark, Menarini, Merck, Mundipharma, Novartis, Pfizer, Pulmatrix, Teva, Theravance, and Verona, outside the submitted work.
Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius, and Paul Dorinsky are employees of AstraZeneca and hold stock and/or stock options in the company.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by AstraZeneca. The funder of the study had a role in study design, data collection, data analysis, data interpretation, and writing of the report. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Data availability: Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
ORCID iD: Klaus F Rabe  https://orcid.org/0000-0002-7020-1401
https://orcid.org/0000-0002-7020-1401
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Klaus F. Rabe, LungenClinic Grosshansdorf and Christian-Albrechts University Kiel, Airway Research Center North, Member of the German Center for Lung Research (DZL), Grosshansdorf 22927, Germany.
Fernando J. Martinez, Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medicine, New York, NY, USA
Dave Singh, Medicines Evaluation Unit, University of Manchester, Manchester University NHS Foundation Trust, Manchester, UK.
Roopa Trivedi, AstraZeneca, Durham, NC, USA.
Martin Jenkins, AstraZeneca, Cambridge, UK.
Patrick Darken, AstraZeneca, Gaithersburg, MD, USA.
Magnus Aurivillius, AstraZeneca, Gothenburg, Sweden.
Paul Dorinsky, AstraZeneca, Durham, NC, USA.
References
- 1.World Health Organization. Global health estimates 2016: deaths by cause, age, sex, by country and by region 2000–2016. https://www.who.int/healthinfo/global_burden_disease/estimates/en/ (2018; accessed 11 April 2019).
- 2.World Health Organization. Global health estimates: life expectancy and leading causes of death and disability. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (2020; accessed January 6 2020).
- 3.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.0-16Nov20_WMV.pdf (2021; accessed 18 November 2020).
- 4.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burge S, Wedzicha JA.COPD exacerbations: definitions and classifications. Eur Respir J 2003; 21: 46s–53s. [DOI] [PubMed] [Google Scholar]
- 7.Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med 2020; 383: 35–48. [DOI] [PubMed] [Google Scholar]
- 8.Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for COPD: a randomized, double-blind, multi-center parallel-group study. Am J Respir Crit Care Med 2021; 203: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med 2018; 6: 747–758. [DOI] [PubMed] [Google Scholar]
- 10.Rabe KF, Fabbri LM, Martinez FJ, et al. Seasonal variation in COPD exacerbations: a post-hoc analysis from the KRONOS phase III study of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler (BGF MDI). Am J Respir Crit Care Med 2020; 199: A1571. [Google Scholar]
- 11.Rabe KF, Martinez FJ, Ferguson GT, et al. A phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6 μg and 160/18/9.6 μg using co-suspension delivery technology in moderate-to-very severe COPD: the ETHOS study protocol. Respir Med 2019; 158: 59–66. [DOI] [PubMed] [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 13.Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 2016; 387: 1817–1826. [DOI] [PubMed] [Google Scholar]
- 14.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008; 178: 332–338. [DOI] [PubMed] [Google Scholar]
- 15.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med 2018; 378: 1671–1680. [DOI] [PubMed] [Google Scholar]
- 16.Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med 2018; 6: 117–126. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet 2018; 391: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 19.Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015; 3: 435–442. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Roisin R, Rabe KF, Vestbo J, et al. Global initiative for Chronic Obstructive Lung Disease (GOLD) 20th anniversary: a brief history of time. Eur Respir J 2017; 50: 1700671. [DOI] [PubMed] [Google Scholar]
- 21.Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet 2016; 388: 963–973. [DOI] [PubMed] [Google Scholar]
- 22.Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med 2015; 163: 461–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-6-tar-10.1177_17534666211034329 for Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial by Klaus F. Rabe, Fernando J. Martinez, Dave Singh, Roopa Trivedi, Martin Jenkins, Patrick Darken, Magnus Aurivillius and Paul Dorinsky in Therapeutic Advances in Respiratory Disease